Validation of liquid chromatography mass spectrometry (LC-MS) methods
8.2 Examples of unstable analytes
Examples of unstable analytes
http://www.uttv.ee/naita?id=23671
https://www.youtube.com/watch?v=Gn8Wde6lD-w&feature=youtu.be
Table 1. Examples of unstable analytes
|
Compound |
Typical cause of instability |
|
Acyl glucoronide metabolites |
Hydrolysis to parent drug, acyl migration [] |
|
Lactones |
Reversible hydrolysis to hydroxyl acid [] |
|
Prodrugs |
Conversion into active drug by ester bond cleavage [] |
|
Amides |
Hydrolysis to acid [] |
|
Oxidizable compounds |
Oxidative degradation
(e.g. alcohol to ketone or aldehyde [], dobutamine []) |
|
Cytostatic nucleosides |
Enzymatic deamination [] |
|
Enantiomers |
Racemization [] |
|
Cis/trans isomers |
Cis-trans interconversion [] |
|
Beta-lactams [, , ] (all penicillin derivatives) |
Hydrolysis, enzymatic degradation, oxidation, presence of metal ions |
In one example, the bench-top/short term of the beta-lactam antibiotic – meropenem – in processed blood plasma samples at the room temperature (+20 °C) was investigated during 24 h using overnight injections and after 12 h the concentration of meropenem in the samples was only 17% of the original concentration at both concentration levels 1 µg/mL and 25 µg/mL. After keeping the autosampler at 4 °C and samples stored at -20 °C until loading into the autosampler, the stability (ST%) (see section 8.3) remained around 95% after 3 h and 92 % after 12 h storage in an autosampler (+4 °C) []. Thus, storage of samples and solutions at low temperature is a powerful means of increasing stability.
In another example [], the stability of Penicillin G was examined over a 16 h time-period and also compared in different pH conditions since Penicillin G is well known for its instability in solutions and the stability of penicillins is also highly dependent on the pH of the solution. The bench-top/short term stability of the Penicillin G in the processed blood plasma samples in an autosampler thermostated at +4 °C showed rapid degradation: ST% was 20% after 16 h for samples with a pH 6.2. After the pH adjustment to 7.0, processed blood plasma samples showed significant improvement on ST%, resulting in 78% after 16 h at the +4 °C. This example convincingly demonstrates how small changes in conditions can strongly influence an analyte stability.
Moreover, microbial contamination can lead to the degradation of the analyte as it was observed for two biomarkers thymidine and 2′-deoxyuridine in urine samples. No analytes were detected in urine after storage at ambient temperature for 24 h and at 4 °C for 14 days []. The stability of samples was improved with the usage of perchloric acid.
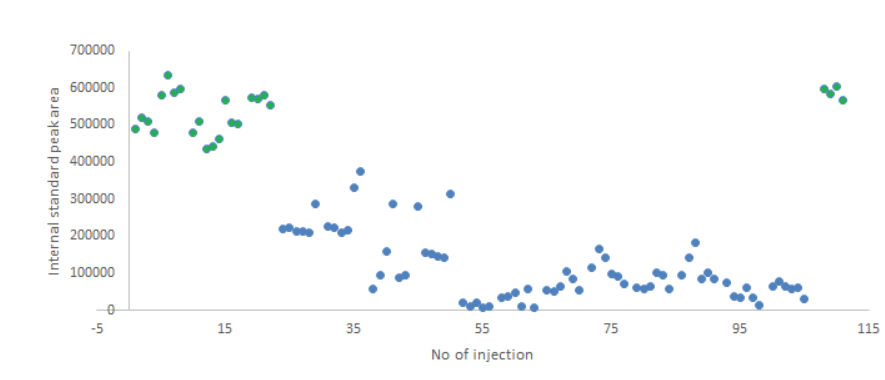
Oxidation of dobutamine was observed when the stability of plasma samples were tested. After the addition of the ascorbic acid as the stabilizer to the plasma samples, a significantly increased sample stability has been onserved []. Interestingly, the internal standard of dobutamine (dobutamine-D4) indicated a decrease of peak area over 115 injection in samples where ascorbic acid was not added as a stabilizer. A stabilizer helped clearly to maintain the concentration of an analyte and an internal standard’s concentration in the processed sample. However, this also means that suitable internal standards could potentially help to overcome analyte stability issues in case degradation of internal standard mimics the degradation of an analyte and additional analyte degradation would not take place before the addition of internal standard.