
Validation of liquid chromatography mass spectrometry (LC-MS) methods
8.3 Evaluation of stability
The time during which the is tested, is important, but it is usually not specified in the guidelines and only brief recommendations are given. The reason being that depending on the particular situation, the suitable duration of the stability study can be very different and should be decided by the analyst based on the situation at hand. Usually when the stability of the analyzed sample solutions or standard solutions is evaluated, the injections of the sample solutions and standard solutions are carried out overnight (i.e. the duration of the study is 10-20 h). Testing of not yet processed samples should be carried out covering the actual duration of the sample storage during the proposed research study. Analyte concentration should be evaluated over a specific storage period and in conditions that would improve the stability over time, should be identified.
The experimental design of the stability testing should take into account. The most important parameters – storage time and temperature. Stability results should be used as the indicator to adjust one or the other in order to improve the stability of the analyte or internal standard.
Stability should be studied at least at two concentration levels – low and high concentration level and in a matrix matching the “real-life” matrix.
For doing this, the native blank biological matrix should be used and the analyte should be spiked into the matrix at these concentration levels.
Bench-top stability or short-term stability at room temperature or sample processing temperature will indicate the analyte stability under sample preparation conditions. Freeze-thaw stability is usually evaluated during three thawing cycles to predict the influence of possible delays and glitches on the sample handling.
Stability should be evaluated at different time points and samples should be analyzed in six [] or miminum of three [, ] replicates. However, EU regulation 2021/808 [] finds evaluation from at least five replicates sufficient.
The analyte or internal standard stability in the test or reference solutions, ST%, expresses the part of the analyte or internal standard in a sample that does not decompose before the actual LC-MS analysis of the sample. In the absence of decomposition, ST% = 100%.
Evaluation of stability
http://www.uttv.ee/naita?id=23670
https://www.youtube.com/watch?v=ClUMsRPgmOY&feature=youtu.be
Stability can be evaluated either via chromatographic peak areas (a) or via concentrations (b)
(a) Stability can be evaluated via peak areas as follows:
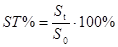 (Eq 1)
(Eq 1)
where S0 is the initial peak area, determined without introducing any extra pauses in the analysis process; St is the peak area obtained when analysis is carried out with making a pause with duration t in the analysis.
(b) Stability can be evaluated via concentrations as follows:
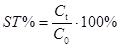 (Eq 2)
(Eq 2)
where C0 is the initial concentration, determined without introducing any extra pauses in the analysis process; Ct is the concentration obtained when analysis is carried out with making a pause with duration t in the analysis.
Stability can be eventually expressed as the average ST% value of analyte found in the sample under specific conditions. The freshly prepared calibration standards (at similar concentration levels as the stability study samples) are considered as containing 100% of the initial analyte content.
Stability evaluation according to EU regulation 2021/808 [] includes comparison of the mean value from five replicates of stored samples compared to the mean value of freshly prepared samples, the difference between two shall not be larger than 15%.
Moreover, stability can also be evaluated as %nominal of QC samples [, ]. Analytes are considered to be stable stability remains within +/-15% [, ] of analyte’s nominal concentration.


