MOOC: Validation of liquid chromatography mass spectrometry (LC-MS) methods (analytical chemistry) course
5.4 Quantitative estimation of matrix effect, recovery and process efficiency
Quantitative estimation of the ionization suppression is possible with post-extraction addition methods as is explained in the following videos. The first video explains the principles of evaluating a matrix effect and also touches upon its relations with and process efficiency:
Calculating matrix effect, recovery and process efficiency
http://www.uttv.ee/naita?id=23248
https://www.youtube.com/watch?v=J1O1bD7gEy4
The second video explains evaluating a matrix effect, recovery and process efficiency in practice:
Practical evaluation of matrix effect, recovery and process efficiency
http://www.uttv.ee/naita?id=23476
https://www.youtube.com/watch?v=vwRVkhZ8GiY
For this approach, the analyte standard solution with a known concentration is prepared in the solvent and analyzed with LC-ESI-MS giving the peak area (signal) Sstandard. Also a blank sample extract is prepared and spiked with the analyte at the same concentration level and ia thereafter analyzed giving the peak area (signal) Ssample. The ionization suppression/enhancement effect can be calculated:
 (Eq 1)
(Eq 1)
MEionization value of 100% indicates no effect, less than 100% indicates an ionization suppression and MEionization over 100% indicates an ionization enhancement due to the coeluting sample compounds. From this definition, though most often used in the LC-MS literature, some possible misunderstandings can arise. The expression “reduce matrix effect” does not mean reduced value of %ME, but a MEionization value becoming closer to 100%.
Calculating matrix effect based on signals
http://www.uttv.ee/naita?id=24822
https://www.youtube.com/watch?v=XZALDnUV9xs&t=32s
Calculating matrix effect based on concentrations
http://www.uttv.ee/naita?id=24818
https://youtu.be/3B9sdDoGx18?si=XwojR_j872wDGx7T
Calculating matrix effect based on slopes
http://www.uttv.ee/naita?id=24821
https://www.youtube.com/watch?v=WJ9-O4gJxlk
Sometimes also the positive/negative MEionization scale is used, where 0% denotes no effect, values above 0% indicate an ionization enhancement and below 0% a suppression. The corresponding equation is:
 (Eq 2)
(Eq 2)
Instead of comparing the peak areas, calibration graph slopes can be compared [].
A similar approach is described in the most recent validation guideline. In this approach, two calibration graphs are constructed, one in the solvent and the other one in the post-extraction spiked samples (i.e. sample extracts obtained from sample preparation). This approach is usable also in the case when blank matrix is unavailable.
Several aspects have to be kept in mind:
(a) The intercepts of both calibration graphs have to be negligible so that the ionization suppression/enhancement would not depend on the analyte concentration. Unfortunately, the latter is not always true.
(b) Before using an approach based on the slope, of the method needs to be studied. In the literature, this approach for ionization suppression/enhancement is often used and sometimes also combined with F– and t-test or ANOVA to evaluate the statistical significance of the obtained matrix effect values.
(c) All of the calculations described above can be done either in the signal scale or concentration scale. The obtained results are fairly similar if the samples used for the ionization suppression/enhancement study are within the and the intercept of the calibration graph is negligible. If these requirements are not fulfilled, it is more useful, from the method point of view, to use the concentration-based calculations.
If signal- or concentration-based calculations are used (not slope-based), the number of samples and replicates used for the suppression/enhancement assessment during validation becomes an issue. Often several replicates are at one or more concentration levels.
(d) It has been often shown that matrix effects depend on the sample source (eg different variety of fruit []). It is therefore also recommended to use different matrices for suppression/enhancement evaluation. In the literature the number of matrices used varies a lot.
(e) In the literature [, ] it has been observed that ionization suppression/enhancement may strongly vary from day to day and it cannot be estimated once during the method optimization/validation and then be used later for result correction.
Reducing matrix effect
Reducing matrix effect
http://www.uttv.ee/naita?id=23288
https://www.youtube.com/watch?v=Oh3eZKYpa6g
Whenever possible and practical, ionization suppression (matrix effect) should be eliminated or significantly reduced. If it is not possible to reduce the ionization suppression to the level of being insignificant, it should be taken into account when calculating the results. Several approaches have been suggested and tested for reducing the ionization suppression effect, mainly focusing on the ESI ionization source. In broad terms the approaches can be categorized as based on (a) the sample preparation, (b) the instrumental modifications and (c) the modifications in LC method:
(a) Less than ideal sample preparation may be viewed as the main reason of occurrence of ionization suppression. In case of a perfect sample preparation combined with the perfect chromatographic separation – leading to the chromatogram where the analyte is completely separated from all of the matrix components – ionization suppression would not occur and would not have to be considered. Unfortunately, perfect sample preparation methods are not available in most cases. A number of literature sources address choosing the most effective sample preparation method from the matrix effect point of view. In LC-MS solid phase extraction (SPE), liquid-liquid extraction (LLE), precipitation/centrifugation or combinations of these as well as other methods are used for the sample preparation.
Different sample preparation techniques have been compared and for example found that for phenacetin and caffeine determination in endogenous plasma, protein precipitation is the least favourable technique for LC-ESI-MS analyses while LLE was the most favourable []. Additionally, LLE has been found to be more effective sample preparation technique than SPE for methadone determination, because the latter tends to concentrate not only the analyte but also the matrix compounds similar to the analyte (i.e. potentially co-eluting from HPLC with the analyte) [ref 30]. The reason probably being that for LLE a larger selection of extracting solvents is available and therefore more freedom in varying is achievable. On the other hand, in the case of SPE, a solid phase similar to the HPLC stationary phase is often used (often both are low polarity C18 or C8 phases) and therefore a little additional/different selectivity is obtained during sample preparation. Additionally, it has been shown that sample pre-concentration may significantly increase ionization suppression.
(b) The main instrumental modification that can be considered is using a non-ESI ion source, such as APCI instead of ESI, since ionization in the APCI source has been demonstrated to be less affected by the matrix effects [, , , ]. Also, switching the ESI source from positive to negative ionization mode or reducing the flow rate of the effluent have also been demonstrated to be efficient in some cases []. Unfortunately, there are numerous analytes for which neither the use of APCI nor switching to negative mode ESI are suitable. Furthermore, among the different LC-MS ion sources, ESI in general tends to have the lowest limits of detection [].
(c) The two main LC-method-related matrix effect reduction possibilities are improvement of the chromatographic separation, e.g. with ultra-high performance liquid chromatography (UPLC/UHPLC), and sample dilution. Both have been used by numerous authors. Dilution has been shown to significantly reduce the ionization suppression []. However, it is often impossible to dilute the sample sufficiently so that the ionization suppression will completely disappear, because the analyte concentration may fall below the limit of quantification. In such cases, the so-called extrapolative dilution approach [ref 37] has been found useful, which consists in diluting the sample as far as possible and if the suppression is still present then extrapolating the analyte concentration mathematically to infinite dilution.
The following video presents a practical discussion on these issues:
Accounting for matrix effect
http://www.uttv.ee/naita?id=23308
https://www.youtube.com/watch?v=5lonQCdmcis
Sometimes it is too difficult (and therefore impractical) or impossible to remove all of the matrix effect, therefore, approaches accounting for the matrix effect have also been developed. Most of them fall either into the category of internal standard usage or matrix-matched calibration [].


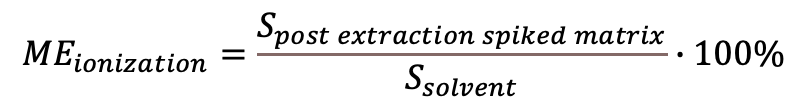 (Eq 1)
(Eq 1)
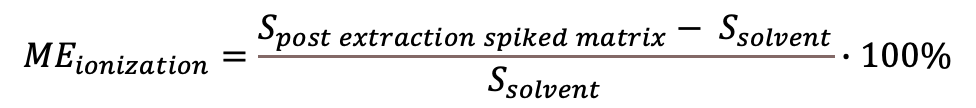 (Eq 2)
(Eq 2)