MOOC: Validation of liquid chromatography mass spectrometry (LC-MS) methods (analytical chemistry) course
2.7. Identity confirmation examples
Example 1
It is the basic assumption of liquid chromatography, that the retention time of an analyte is the same on the chromatograms of standard solutions and sample solutions. Two validation guidelines have set a clear criteria for the retention time tolerance: 2002/657/EC [] and SANTE/SANCO []. 2002/657/EC limits the tolerance of the relative retention time to 2.5% and the new SANTE requires 0.1 min absolute retention time tolerance (the previous SANCO limit was 0.2 min).
Assessment of retention time deviation according to SANTE.
Analysis of pharmaceutical residues in the sewage sludge compost was carried out. The retention times of the three analytes in the calibration solutions are presented in Table 1 along with the average retention times for each analyte.
Table 1. Experimental and average retention times (in minutes) of metformin, diclofenac and carbamazepine in calibration solutions.
|
Metformin |
Diclofenac |
Carbamazepine |
|
|
Cal 1 |
7.675 |
19.172 |
21.263 |
|
Cal 2 |
7.675 |
19.162 |
21.242 |
|
Cal 3 |
7.664 |
19.172 |
21.263 |
|
Cal 4 |
7.675 |
19.172 |
21.253 |
|
Cal 5 |
7.664 |
19.161 |
21.252 |
|
Cal 6 |
7.664 |
19.161 |
21.252 |
|
Cal 7 |
7.664 |
19.172 |
21.263 |
|
Average (RT): |
7.669 |
19.168 |
21.255 |
The average retention times in calibration solutions are used as reference points to assess the deviations of retention times of analytes in sample solutions. Table 2 presents experimental retention times of analytes in sewage sludge compost extracts. The difference of a retention time from the respective reference value is calculated. Absolute values of this difference must not exceed the tolerance limit set by SANTE (0.1 min). Calculated differences and assessment are also included in Table 2.
Table 2. Retention times of analytes (in minutes) in sewage sludge compost extract sample chromatograms. Differences of retention times from respective reference values are presented along with assessment of the result with respect to the SANTE 0.1 min tolerance limit.
|
|
Metformin |
Diclofenac |
Carbamazepine |
||||||
|
RT |
Difference |
Complies? |
RT |
Difference |
Complies? |
RT |
Difference |
Complies? |
|
| Sample 1 |
7.598 |
-0.071 |
Yes |
19.063 |
-0.105 |
No |
21.23 |
-0.025 |
Yes |
| Sample 10 |
7.566 |
-0.103 |
No |
19.183 |
0.015 |
Yes |
21.285 |
0.03 |
Yes |
| Sample 20 |
7.577 |
-0.092 |
Yes |
19.139 |
-0.029 |
Yes |
21.263 |
0.008 |
Yes |
| Sample 30 |
7.533 |
-0.136 |
No |
19.194 |
0.026 |
Yes |
21.296 |
0.041 |
Yes |
| Sample 40 |
7.544 |
-0.125 |
No |
19.183 |
0.015 |
Yes |
21.285 |
0.03 |
Yes |
| Sample 50 |
7.522 |
-0.147 |
No |
19.194 |
0.026 |
Yes |
21.285 |
0.03 |
Yes |
| Sample 55 |
7.435 |
-0.234 |
No |
19.172 |
0.004 |
Yes |
21.274 |
0.019 |
Yes |
From Table 2 it is evident that all carbamazepine results and all but one diclofenac results comply with SANTE requirement. But the situation is different with metformin – only two results comply. A study of the metformin retention time differences reveals that all the differences are negative. This indicates that some systematic effect is present. In some cases, the sample matrix affects the retention. Matrix matched calibration could then solve the problem. It is also possible, that the contamination gradually builds up in the column and alters the stationary phase properties and consequently the retention – more extensive clean-up of the sample extracts could help.
In conclusion, SANTE retention time tolerance check can be rather restrictive, but it is also helpful for diagnosing possible problems in analytical methods.
Assessment of retention time deviation according to 2002/657/EC.
The validation guideline 2002/657/EC by the European Commission uses the concept of relative retention time (RRT) for establishing the tolerance limit of retention time. The RRT is defined as a ratio of retention time of the analyte (RT) to the retention time of the internal standard (IS). For this example, metformin can be regarded as a retention time internal standard. Experimental data obtained from calibration solutions along with RRT values are presented in Table 3. The averages of RRT-s obtained from calibration solutions serve as reference values for analytes.
Table 3. Absolute (RT) and relative (RRT) experimental retention times obtained from calibration solutions. Metformin is used as an internal standard (IS). Average relative retention times of diclofenac and carbamazepine.
|
Metformin (IS) |
Diclofenac |
Carbamazepine |
|||
|
RT |
RT |
RRT |
RT |
RRT |
|
| Cal 1 |
7.675 |
19.172 |
2.498 |
21.263 |
2.770 |
| Cal 2 |
7.675 |
19.162 |
2.497 |
21.242 |
2.768 |
| Cal 3 |
7.664 |
19.172 |
2.502 |
21.263 |
2.774 |
| Cal 4 |
7.675 |
19.172 |
2.498 |
21.253 |
2.769 |
| Cal 5 |
7.664 |
19.161 |
2.500 |
21.252 |
2.773 |
| Cal 6 |
7.664 |
19.161 |
2.500 |
21.252 |
2.773 |
| Cal 7 |
7.664 |
19.172 |
2.502 |
21.263 |
2.774 |
|
Average (RRTRef): |
2.499 |
2.772 |
|||
Similarly to calibration data, RRT values are now calculated for all analyte peaks in samples (Table 4). To compare RRT values in sample to a reference value, a relative difference (as percent) is calculated as follows:
 (Eq 1)
(Eq 1)
where RRT is a relative retention time of an analyte in a sample injection and RRTRef is an average of calibration solution RRT-s.
Unsigned value of Rel.Diff is compared to the tolerance limit set by 2002/657/EC: 2.5%. Results of this assessment are also presented in Table 4.
Table 4. Absolute (RT) and relative (RRT) experimental retention times with respect to the internal standard (IS) in sewage sludge compost extract sample chromatograms. Relative differences (as percentage) of relative retention times from respective reference values are presented along with assessment of the result with respect to the 2002/657/EC tolerance limit of 2.5%.
|
Metformin (IS) |
Diclofenac |
Carbamazepine |
|||||||
|
RT |
RT |
RRT |
Rel. Diff. |
Complies? |
RT |
RRT |
Rel. Diff. |
Complies? |
|
| Sample 1 |
7.598 |
19.063 |
2.509 |
0.40% |
Yes |
21.23 |
2.794 |
0.80% |
Yes |
| Sample 10 |
7.566 |
19.183 |
2.535 |
1.40% |
Yes |
21.285 |
2.813 |
1.50% |
Yes |
| Sample 20 |
7.577 |
19.139 |
2.526 |
1.10% |
Yes |
21.263 |
2.806 |
1.20% |
Yes |
| Sample 30 |
7.533 |
19.194 |
2.548 |
1.90% |
Yes |
21.296 |
2.827 |
2.00% |
Yes |
| Sample 40 |
7.544 |
19.183 |
2.543 |
1.70% |
Yes |
21.285 |
2.821 |
1.80% |
Yes |
| Sample 50 |
7.522 |
19.194 |
2.552 |
2.10% |
Yes |
21.285 |
2.83 |
2.10% |
Yes |
| Sample 55 |
7.435 |
19.172 |
2.579 |
3.20% |
No |
21.274 |
2.861 |
3.20% |
No |
According to Table 4 data, almost all retention times are compliant. Only retention times of analytes in the last sample were found to be non-compliant. A reason for this is the retention time of the IS, which is smaller than expected.
Compared to tolerance limits set by SANTE, these of 2002/657/EC appear to be more lenient. IS is required for retention time checking by 2002/657/EC, which makes the approach slightly less comfortable to use.
Example 2
SANTE/SANCO criteria for identification and identification points of 2002/657/EC.
Requirements for number and type (molecular, adduct or product ion) of the monitored ions in mass spectrometry are presented in Table 3 of SANTE/SANCO validation guidelines. For example, the signal from at least two ions must be recorded with any mass spectrometer operated in MS/MS mode regardless of whether mass spectrometer is of low or high resolution. For further requirements see Table 3 in SANTE/SANCO guidelines [ref 4].
The mass spectrometric identification rules used by 2002/657/EC [ref 5] are more elaborate and based on the identification points. The required number of identification points depends on the type of the analyte (see [ref 5] for full details): 4 points are required for substances having anabolic effect and unauthorized substances; 3 points are required for veterinary drugs and contaminants.
Table 5 presents numbers of identification points earned per ion for different MS techniques.
Table 5. Identification points earned for MS techniques according to 2002/657/EC.
|
MS technique |
Identification points earned per ion |
| Low resolution (LR) MS |
1.0 |
| LR-MSn precursor ion |
1.0 |
| LR-MSn transition products |
1.5 |
| High resolution MS |
2.0 |
| High resolution MSn precursor ion |
2.0 |
| High resolution MSn transition product |
2.5 |
In addition to the data in Table 5, there are two additional rules – each ion may be counted only once and the “transition product” includes all generations of transition products. Few examples:
– Unit resolution MS operated in SIM mode and three ions recorded – 3 points.
– Triple quadrupole MS (unit resolution, MS/MS) recording 2 products ions for the same precursor – 4 points (1*1.0+2*1.5=4).
- For example, transitions 142.1→94.1 and 142.1→112.2 for methamidophos (pesticide) analysis.
– LC-MS3 experiment with an ion trap MS (unit resolution) recording 2 product ions – 5.5 points (1.0+1.5+2*1.5=5.5).
– Combined quadrupole-TOF MS with low resolution precursor ion and 2 high resolution product ions – 6 points (1.0+2*2.5=6.0)
Example 3
Calculation and assessment of ion ratios.
SANTE/SANCO and 2002/657/EC limit ion ratio tolerances according to Table 6.
Table 6. Ion ratio tolerances of SANTE/SANCO and 2002/657/EC.
|
Ion ratio |
Relative tolerance (SANTE/SANCO) |
Relative tolerance (2002/657/EC) |
| > 0.5 | ± 30% | ± 20% |
| 0.20 – 0.50 | ± 30% | ± 25% |
| 0.10 – 0.20 | ± 30% | ± 30% |
| < 0.10 | ± 30% | ± 50% |
The ion ratio is calculated as an intensity (or peak area) ratio of a less intense ion to that of a more intense ion. The reference ion ratio value is calculated as an average of ion ratios of calibration solutions. Table 7 illustrates the process of calculating such a reference ion ratio on the example of propamocarb (pesticide) calibration.
Table 7. Example of reference ion ratio calculation based on peak areas (S1, S2) of two MRM transitions.
| Propamocarb | 189→102 | 189→144 | Ion ratio |
| S1 | S2 | S2/S1 | |
| Cal. solution 1 |
555 |
259 |
0.47 |
| Cal. solution 2 |
1176 |
499 |
0.42 |
| Cal. solution 3 |
1707 |
803 |
0.47 |
| Cal. solution 4 |
2404 |
991 |
0.41 |
| Cal. solution 5 |
3031 |
1312 |
0.43 |
| Average: |
0.44 |
In a similar manner, ion ratios are calculated for the analysis of samples (Table 8).
Table 8. Calculation of ion ratio from peak areas (S1, S1) of two MRM transitions recorded from sample chromatograms.
| Propamocarb | 189→102 | 189→144 | Ion ratio |
| S1 | S2 | S2/S1 | |
| Sample solution 1 |
821 |
281 |
0.34 |
| Sample solution 2 |
2221 |
1251 |
0.56 |
To assess the compliance of ion ratios in samples, the following equation can be used:
 (Eq 2)
(Eq 2)
Relative difference for Sample 1 is -23% and for Sample 2 it is 27%. Comparison with limits in Table 6 (second row), reveals that the identity of propamocarb in both samples is confirmed by the rules of SANTE/SANCO rules. Regarding 2002/657/EC limits, presence of propamocarb is not confirmed in Sample 2 as 27% > 25%.
Example 4
Selection of product ions.
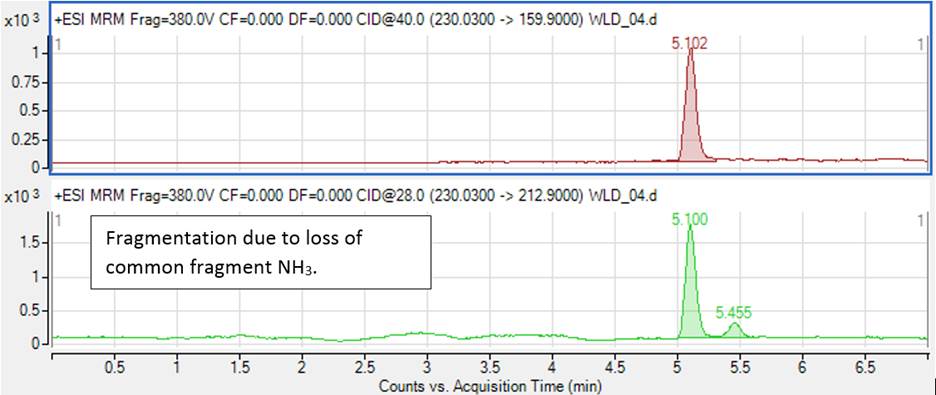
SANTE/SANCO and 2002/657/EC give recommendations for a proper selection of the diagnostic ions. In general, fragmentation due to the loss of common fragments (H2O, NH3 etc) should be avoided, as more background noise and interfering compounds are expected. For example, Figure 1 shows transitions 230→213 and 230→160 registered for clonidine (blood pressure lowering drug). Transition 230→213 is due to the loss of NH3, which leads to a noisier background.
Figure 1. Influence of fragmentation pathways on chromatographic background.
Example 5
How well do all the limitations work?
SANTE/SANCO and 2002/657/EC set rather restrictive rules to a LC-MS analysis. Do all these criteria guarantee unambiguous confirmation of an analyte? Or maybe the rules are too strict?
A method was developed for a tebufenpyrad (pesticide) analysis in a ginger extract. In Figure 2 overlaid chromatograms of the two transitions of tebufenpyrad are presented for a calibration solution and a ginger extract.
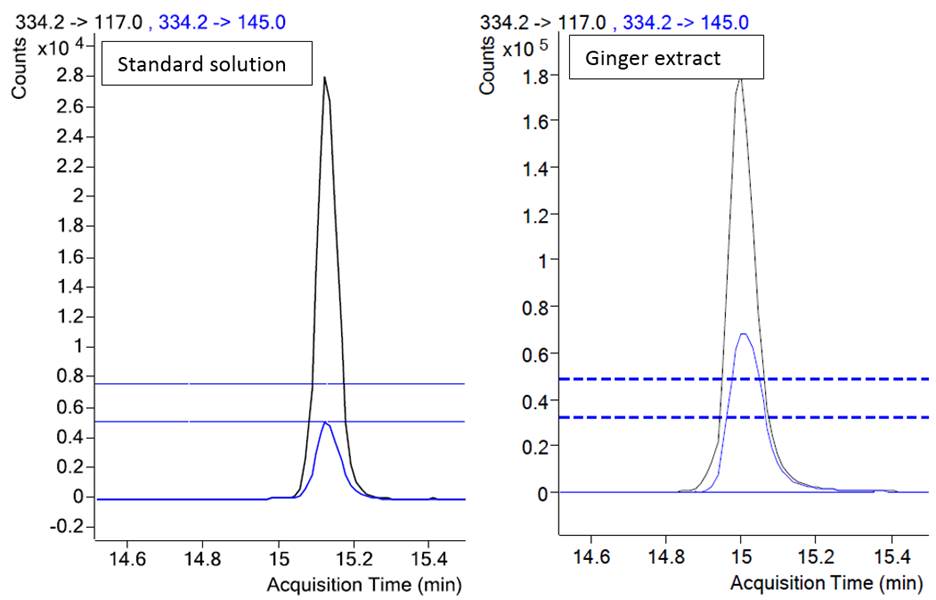
Figure 2. Chromatograms of two transitions of tebufenpyrad in a standard solution and in a sample. (Data from http://www.agilent.com/cs/library/eseminars/public/Triggered%20MRM%20for%20the%20Analysis%20of%20Pesticides.pdf)
Retention times of peaks are similar: 15.1 min in a standard and 15.0 min in an extract and would pass the requirements for the retention time match by SANTE (0.1 min). The ion ratio of the two transitions for a tebufenpyrad in a calibration standard is 0.21 and for peaks in a ginger extract 0.469. The relative difference of ion ratios is 123%, which exceeds the tolerance limits set by SANTE/SANCO and 2002/657/EC. Therefore, the presence of a tebufenpyrad in a ginger could not be confirmed. Indeed, further analysis revealed that it was an endogenous ginger compound, which yielded this peak.
So, thanks to the strictness of the rules a result was avoided.


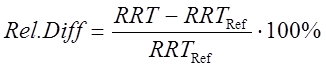 (Eq 1)
(Eq 1)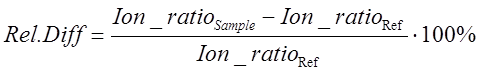 (Eq 2)
(Eq 2)