MOOC: Validation of liquid chromatography mass spectrometry (LC-MS) methods (analytical chemistry) course
6.4. Real life examples
Example of an impure standard
LC-UV method was used for quantification and MS was used for confirmation of the result. A calibration curve was prepared with a separate pure standard of N-nitroso-diphenylamine (N-DPA) and this was used to determine the concentration of a certified standard solution by Dr. Ehrenstorfer. However, instead of a certified 10 ng/mL, a concentration of 7.7 ng/mL was observed for the solution. A closer look at the UV chromatogram showed two peaks for the Dr. Ehrenstorfer solution while the calibration curve solutions had just one peak. The MS spectra shows that the unknown additional peak might possibly be a diphenylamine.
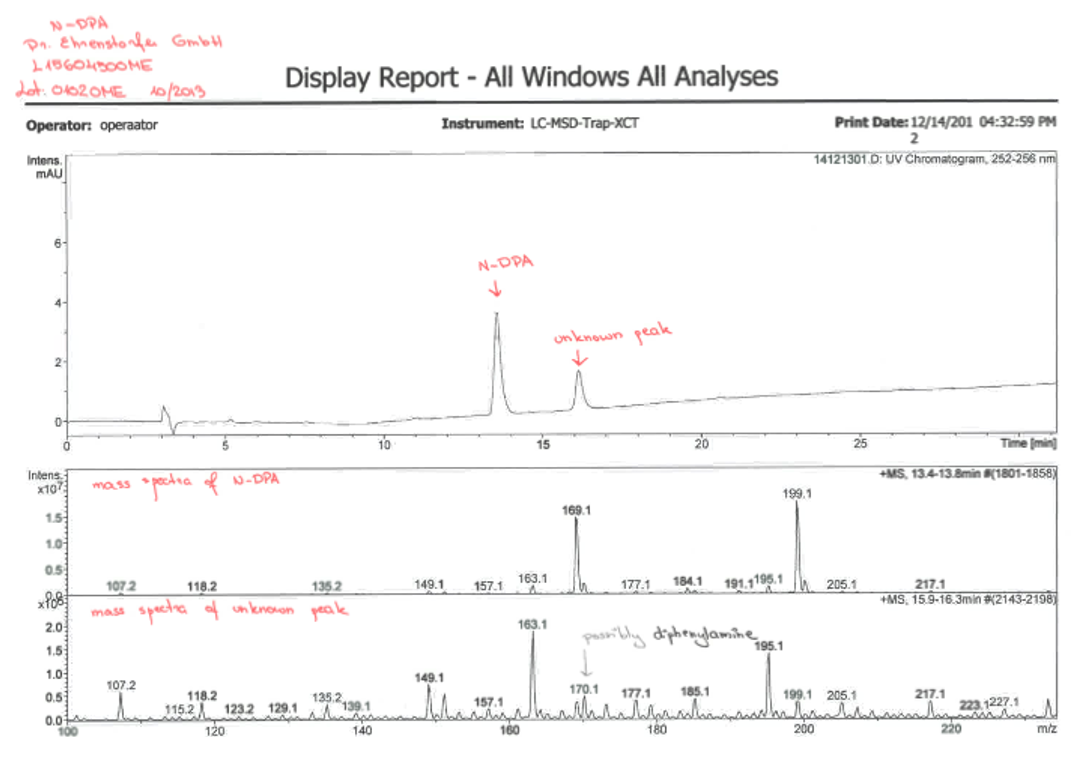
Figure 1. UV chromatogram of Dr. Ehrenstorfer solution and mass spectra of the N-DPA and unknown peaks.
PFOS standards by different producers
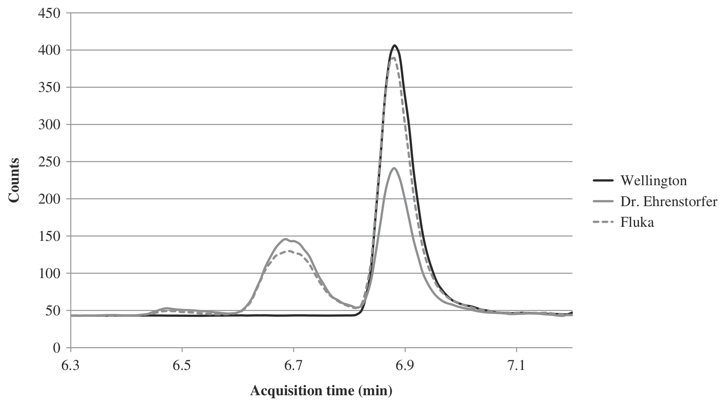
Figure 2. Chromatograms of PFOS solutions of Dr. Ehrenstorfer, Fluka and Wellington Laboratories.
PFOS is an anthropogenic fluorosurfactant and global pollutant. It is known that there can be different isomers for PFOS. During method development, various reference solutions were analysed and the chromatographic analysis revealed that both PFOS commercial standard solutions [Dr. Ehrenstorfer (Lot no 5011ME) and Fluka (Lot no SZB008XV)] contained not only linear PFOS isomer but also a branched version (Figure 2). No indication that the standard is a mixture of isomers is mentioned by these manufacturers in the documentation. A linear PFOS solution from Wellington Laboratories Inc. was used to quantify the amount of linear PFOS in Dr. Ehrenstorfer and Fluka PFOS solutions. []
Problems with isotope labelled internal standards
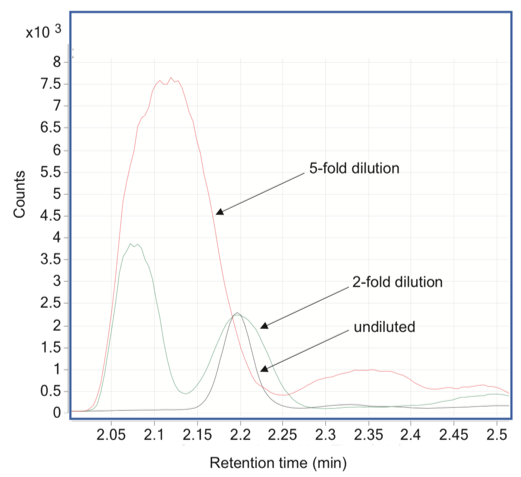
Figure 3. Normetanephrine-D3 (C = 500 pg/mL) extracted ion chromatograms (169->137) of protein precipitated (with acetonitrile) sample extracts analyzed undiluted and with 2- and 5-fold dilutions.
During method validation, an unusual interference for a D3-labeled internal standard of normetanephrine was discovered – a signal of the interfering compound increased while the matrix effects were reduced by dilution, e.g. dilution eliminates the matrix suppression on the interfering compound. The results stress the need to monitor interfering compounds and evaluate matrix effects at every step of the method development. Matrix effects and interferences can be different for analytes and their corresponding isotopically labeled internal standards. This means that the use of isotopically labeled internal standards cannot guarantee of the obtained results. []
The impurities in eluent additive affecting the accuracy
Emodepside is an antiparasitic drug licenced for use in cats. A bioanalytical LC-MS method was developed and validated for measuring emodepside in serum. Eluent composition in an isocratic elution contained 10mM ammonium acetate and acetonitrile 30/70. The initial accuracy and batches demonstrated excellent assay performance when LiChropur™, an eluent additive for LC-MS grade ammonium acetate from Merck, was used. Suddenly, the performance of the assay was compromised, and over 96 injections, the performance of QCs demonstrated a positive increasing throughout the batch and leading to two last sets of QCs failing the acceptance criteria for the bioanalytical method. When the (in)accuracy and precision of the first set of the QCs was around 2-5%, it had increased to 22-27% for the last set of QCs. The same trend was observed over several batches. No change in the analyte and internal standard (deuterated emodepside) peak shape was observed. The troubleshooting led to the finding that the usage of lower-grade ammonium acetate (for HPLC LiChropur™) was in use in problematic batches. Once the ammonium acetate was changed back to the LC-MS grade, the assay performance returned to the level confirmed by the validation. This means that the choice of eluent additive and its purity can have a significant effect on the assay performance, and selection made during assay development/validation has to be respected to ensure the assay performance.

