MOOC: Validation of liquid chromatography mass spectrometry (LC-MS) methods (analytical chemistry) course
8.1 Different types of stability
SANTE/SANCO [] specifies briefly that an analyte in prepared sample extracts has to be evaluated. EU regulation 2021/808 [] outlines the stability requirements for the analyte in the solution and in matrix after short-, medium-, and long-term storage.
Especially in the bioanalytical field, the possible analyte decomposition is of very high importance for the quality of the results and therefore deserves special attention. For this reason, the EMA [], [] and [] validation guidelines specifically address an analyte stability as a separate validation parameter.
The FDA guide [ref 8] distinguishes the following types of stability:
- Freeze and Thaw Stability;
- Bench-Top Stability;
- Long-Term Stability;
- Stock Solution Stability;
- Processed Sample Stability;
- Auto-sampler Stability.
This guideline distinguishes between an analyte stability in the calibration and stock solutions and stability in the sample matrix and stresses the influence of storage conditions, matrix and container system on the stability, in addition to the intrinsic properties of the analyte itself. For example, the analyte stability evaluation in whole blood could be valuable in case an analyte is unstable in whole blood or adsorbs to cellular components during the collection procedure.
According to the EMA [ref 7] guide, a stability of the analyte is evaluated using both low- and high-level QC samples. The investigation of stability should cover:
- Bench-top (short-term) stability in matrix;
- Freeze-thaw stability in matrix;
- Long-term stability in matrix;
- Stability of the analyte in processed samples (storage conditions during analysis and on-instrument/auto-sampler stability);
- Stability of the analyte and IS in stock and working solutions;
- Stability of the analyte in whole blood.
Different types of stability
http://www.uttv.ee/naita?id=23669
https://www.youtube.com/watch?v=po-ZhVfSW8o&feature=youtu.be
The emphasis in the EMA [ref 7] and FDA [ref 8] guides are not as much on the intrinsic stability of the analyte, as on its stability in the specific matrix. EU regulation 2021/808 [ref 5] on the other hand finds both, analyte stability in the solution and in the matrix equally important.
AOAC [ref 9] is less specific on the experiments that have to be carried out and recommends checking the stability of the stock and initial diluted solutions, stored at room or lower temperatures, by repeating their measurements several days or weeks later.
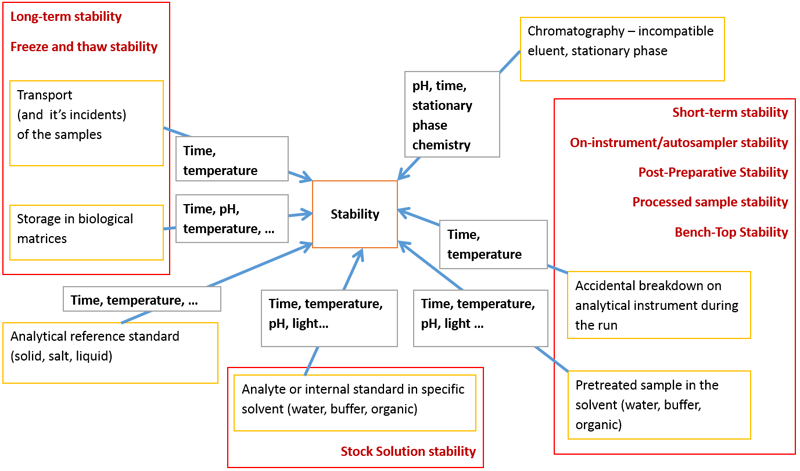
Figure 2. Different types of stability in LC–MS analysis and the chemical/physical parameters influencing stability.
Software  helps to calculate stability in different conditions.
helps to calculate stability in different conditions.

