
Validation of liquid chromatography mass spectrometry (LC-MS) methods
7. Accuracy
– relations to different concepts
http://www.uttv.ee/naita?id=23668
https://www.youtube.com/watch?v=iunClGAivzo
Measurement result accuracy indicates its closeness to the true value []. Accuracy differs from : accuracy can be used to characterize an individual result, but trueness always refers to the mean value of a large number of results. Because of that and because accuracy can characterize an individual result, accuracy involves also . So, the accuracy of a method (i.e. accuracy of the results delivered by the method) is affected by a systematic () as well as random (precision) error components and is, therefore, studied as two components: trueness and precision [].
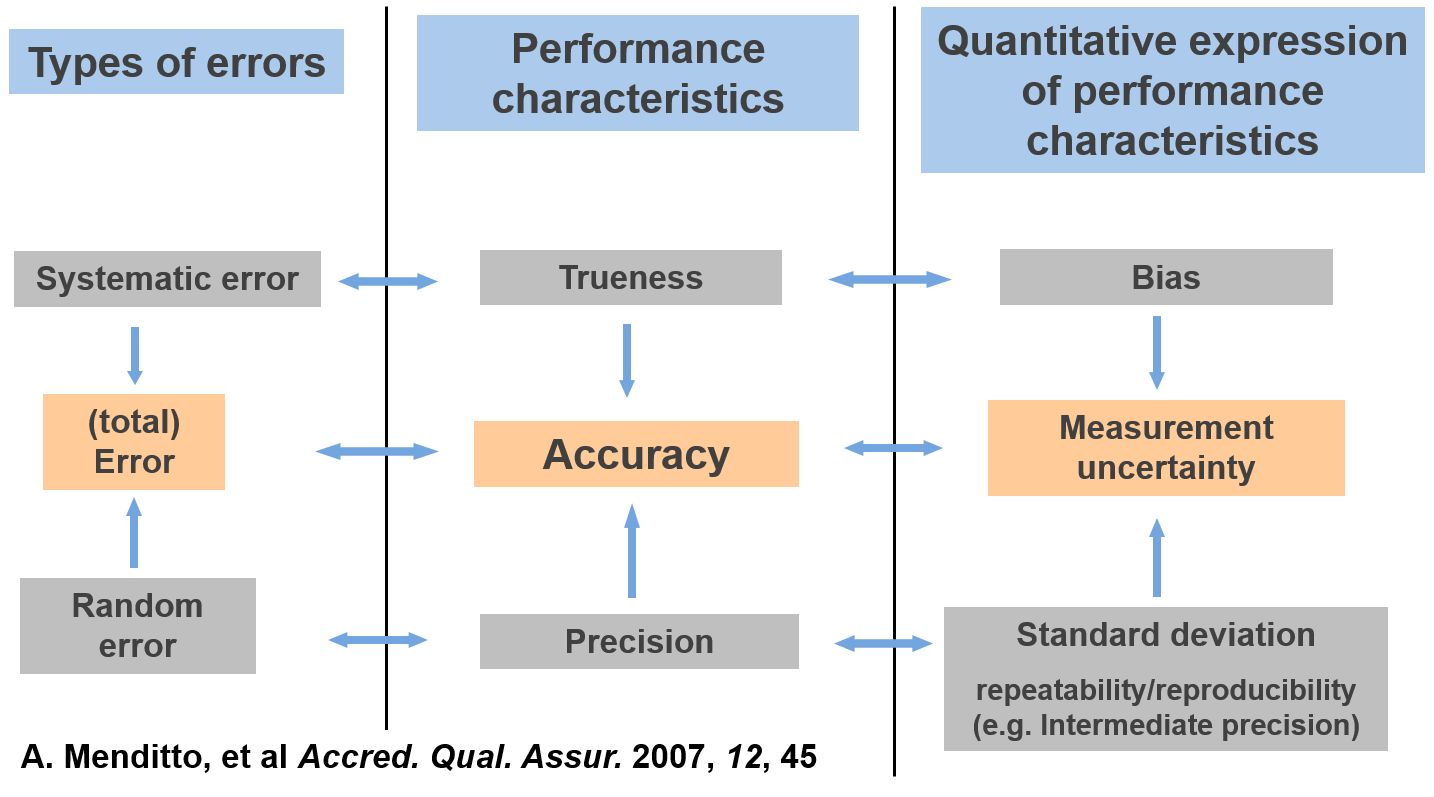
Figure 1. Interrelations between the different error types, the performance characteristics used to estimate them and the ways of expressing the estimates quantitatively. This type of scheme was originally published in ref 56. (See Note 1)
Accuracy, trueness, precision and
http://www.uttv.ee/naita?id=23345
https://www.youtube.com/watch?v=NfEsN1Gaq5k
A number of regulatory bodies (ICH, , EMA) define accuracy as the degree of agreement between the experimental value, obtained by replicate measurements, and the accepted reference value. This definition is identical to the currently accepted definition of trueness. For the evaluation of acceptability of measurement accuracy, different evaluation criteria can be used: En -numbers, z-scores or zeta-scores.
Accuracy is often considered as a qualitative term []. However, in practice it is useful to consider that accuracy is quantitatively expressed as a measurement uncertainty. There are different approaches for the measurement uncertainty estimation, but in practice the approach based on the validation data is often the most convenient. The following video explains the basics of this approach. In-depth treatment of the topic of measurement uncertainty, together with numerous examples and self-tests can be found in the on-line course Estimation of Measurement Uncertainty in Chemical Analysis. Uncertainty estimation on the basis of validation and quality control data is covered in section 10. The Single-lab validation approach.
Measurement uncertainty estimation approaches
http://www.uttv.ee/naita?id=23667
https://www.youtube.com/watch?v=syB2RKAEeMs&t=46s
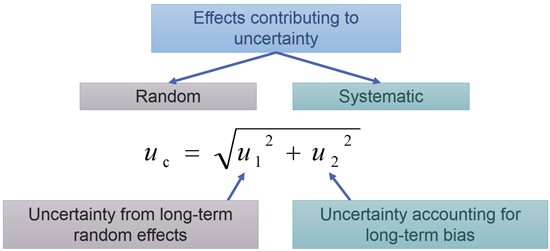
Figure 2. The main idea of estimating measurement uncertainty using validation and quality control data.
***
Note 1: Literature reference in the video has incorrect number of year. The correct reference is as stated here: A. Menditto, et al Accred. Qual. Assur. 2007, 12, 45


