Validation of liquid chromatography mass spectrometry (LC-MS) methods
4. Precision
In the next parts of the course, three important validation parameters (method performance parameters) are introduced and explained: (this section), (section 5) and (section 7). In section 6, different aspects of practical determination of precision and trueness are explored.
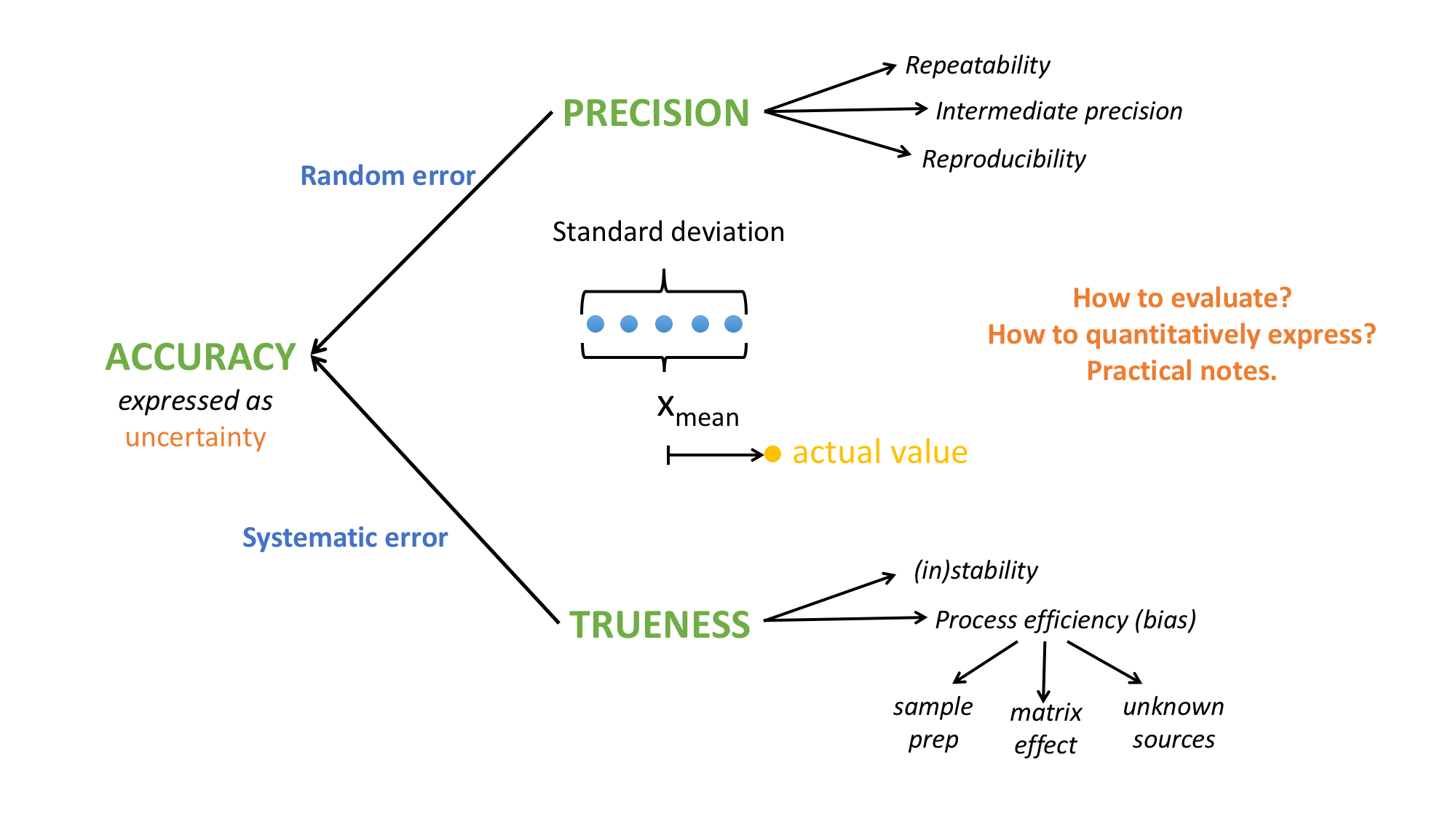
Precision characterizes the closeness of agreement between the measured values obtained by the replicate measurements on the same or similar objects under specified conditions. Precision relates to the random error of a measurement system and is a component of a . Precision can be evaluated with a sample that doesn’t necessarily have to have a known analyte content.
For the sake of completeness, let us also briefly address trueness and accuracy here:
Trueness relates to the systematic error of a measurement system and if rigorously defined, refers to the agreement between the average of infinite number of replicate measured values and the true value of the measured quantity. In practice, trueness is evaluated from a finite, but a reasonably large number of measurements and reference values are used instead of the true value.
Measurement accuracy expresses the closeness of a single measurement result to a reference value. Method validation seeks to investigate an accuracy of the results by assessing both the systematic and random effects (errors) on single results. These errors are caused by a range of reasons, such as the imperfect analyte during sample preparation, possible ionization suppression of the analyte, possible instability of the analyte and others. These errors put together give us the (total) error. Since this error is not experimentally accessible, we operate with the estimates of errors – the performance characteristics.
Accuracy embraces both trueness and precision and can in principle be characterized via precision and trueness. A single-parameter expression of an accuracy is a measurement uncertainty. (Figure 1)
Introduction to precision, trueness and accuracy
http://www.uttv.ee/naita?id=23353
https://www.youtube.com/watch?v=_04F0ViTgPw