
Validation of liquid chromatography mass spectrometry (LC-MS) methods
1.2 Carrying out validation
It is always of importance how to perform validation in the most effective way. Based on the literature data and our own experience, we suggest a possible general sequence of validation in Figure 1.
Note that all terminology, abbreviations used in the course can be found in Glossary.
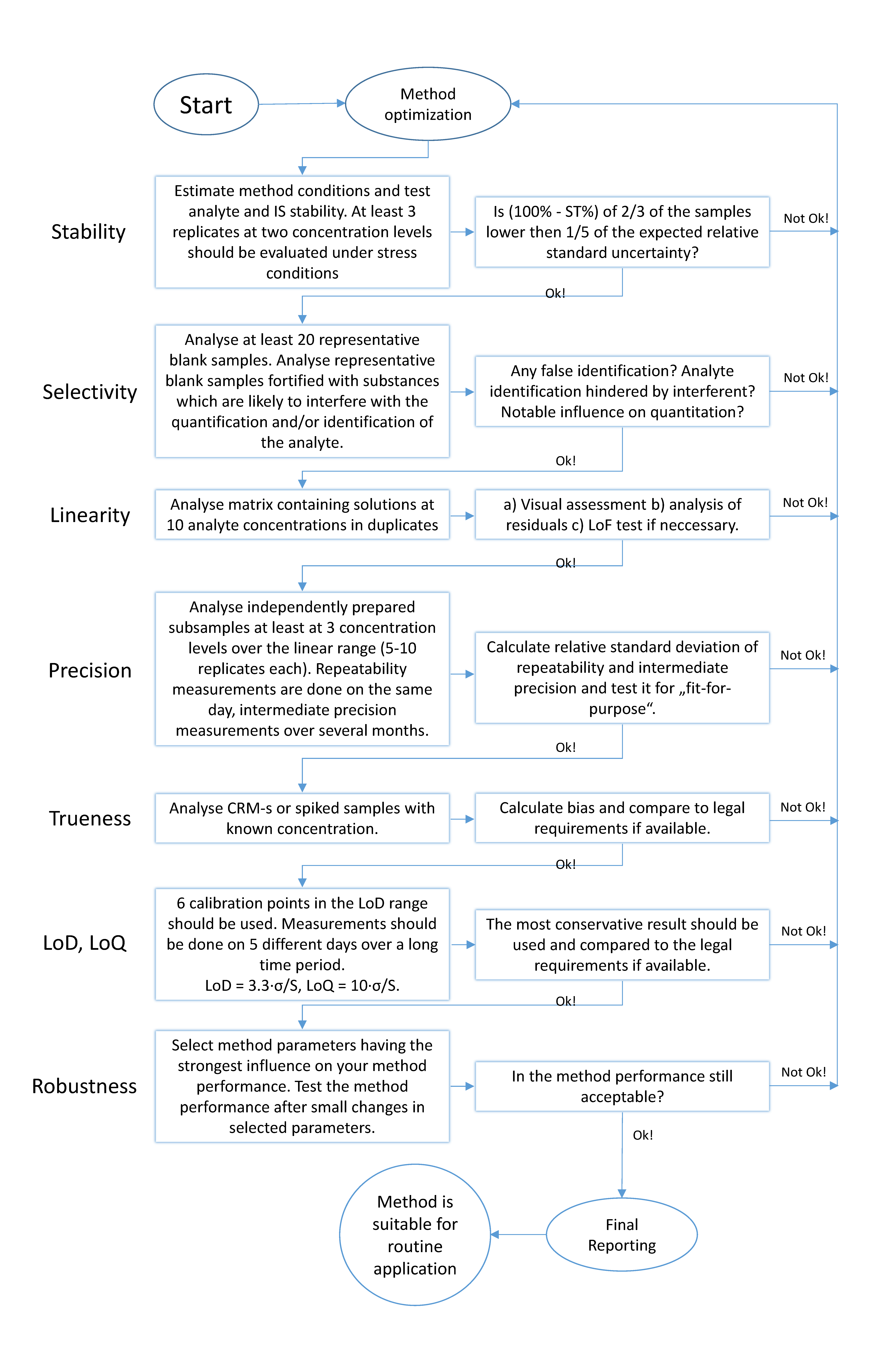
Figure 1. A possible sequence of operations and decisions in LC–MS method validation. All steps are explained in detail in upcoming chapters. ST% refers to stability in per cents, explained in section 8.3. LoF stands for Lack-of-Fit test, explained in 3.3. CRM stands for certified reference material.
Before starting a validation, a clear validation plan is compiled, which consists of the reason for validation, planned experiments, as well as expected outcomes – requirements that need to be met by the method. Requirements often result from guidelines or from other regulatory documents (directives, standards etc.). A validation plan depends on each of the different method under development and it takes into account all the specific aspects related to that method. After carrying out the necessary experiments, a decision must be made if the results are satisfying and consequently if the method is fit for purpose. Validation is documented in a validation report.
Guidelines generally give recommendations how to evaluate each performance parameter separately. At the same time, guidance on how to decide about the whole method’s validation is usually very general: validation has to demonstrate that the values of all evaluated parameters are satisfactory. Few different cases arise.
- (a) When methods are applied in the scope of standards, laws or directives, then requirements from those documents, if present, must be followed and a decision on validation should be based on these documents. When a decision on validation suitability is based on the guidelines, then the decision for each parameter must be given separately according to the requirements.
- (b) Sometimes the client can specify the requirements, then client’s requirements are superior to those in the guidelines.
- (c) If there are no external requirements, then the analyst can set up the requirements himself/herself based on his/her knowledge of the subject.
Validation should start with evaluating analyte and method as all other parameters strongly depend on these. For example, if an analyte extensively decomposes in the autosampler, no linear relation can be achieved. In that case, non-linear calibration models need to be considered. Consequently, we propose an estimation of as the next step, because for an evaluation of and , we need to know the linear/ of the method. We propose studies as the last step of validation. It is sometimes suggested to test robustness as one of the first things in method validation or in the end of the method development phase. We find it important to have some insight, as to which are the most important performance characteristics (e.g. closest to the legal limits or the requirements of the client) before deciding which of the method performance characteristics are varied during the robustness studies.
Not only validation but also an appropriate documentation of the validation is required for an adequate interpretation as well as on the validity of the obtained results. As the last stage of validation, an assessment of validity (fitness for the intended purpose) of the method should be given, based on the validation results.
Overview of validation
http://www.uttv.ee/naita?id=23287
https://www.youtube.com/watch?v=K312lRGTJFk


