Validation of liquid chromatography mass spectrometry (LC-MS) methods
2.7. Identity confirmation examples
Example 1
It is the basic assumption of liquid chromatography, that the retention time of an analyte is the same on the chromatograms of standard solutions and sample solutions. Two validation guidelines have set a clear criteria for the retention time tolerance: 2021/808 [] and SANTE []. Both guidelines require 0.1 min absolute retention time tolerance. If internal standard is used then the relative retention time deviation can be at most 1% (according to 2021/808).
Assessment of retention time deviation according to SANTE and 2021/808.
Analysis of pharmaceutical residues in the sewage sludge compost was carried out. The retention times of the three analytes in the calibration solutions are presented in Table 1 along with the average retention times for each analyte.
Table 1. Experimental and average retention times (in minutes) of metformin, diclofenac and carbamazepine in calibration solutions.
|
Metformin |
Diclofenac |
Carbamazepine |
|
|
Cal 1 |
7.675 |
19.172 |
21.263 |
|
Cal 2 |
7.675 |
19.162 |
21.242 |
|
Cal 3 |
7.664 |
19.172 |
21.263 |
|
Cal 4 |
7.675 |
19.172 |
21.253 |
|
Cal 5 |
7.664 |
19.161 |
21.252 |
|
Cal 6 |
7.664 |
19.161 |
21.252 |
|
Cal 7 |
7.664 |
19.172 |
21.263 |
|
Average (RT): |
7.669 |
19.168 |
21.255 |
The average retention times in calibration solutions are used as reference points to assess the deviations of retention times of analytes in sample solutions. Table 2 presents experimental retention times of analytes in sewage sludge compost extracts. The difference of a retention time from the respective reference value is calculated. Absolute values of this difference must not exceed the tolerance limit set by SANTE and 2021/808 (0.1 min). Calculated differences and assessment are presented in Table 2.
Table 2. Retention times of analytes (in minutes) in sewage sludge compost extract sample chromatograms. Differences of retention times from respective reference values are presented along with assessment of the result with respect to the 0.1 min tolerance limit.
|
|
Metformin |
Diclofenac |
Carbamazepine |
||||||
|
RT |
Difference |
Complies? |
RT |
Difference |
Complies? |
RT |
Difference |
Complies? |
|
| Sample 1 |
7.598 |
-0.071 |
Yes |
19.063 |
-0.105 |
No |
21.23 |
-0.025 |
Yes |
| Sample 10 |
7.566 |
-0.103 |
No |
19.183 |
0.015 |
Yes |
21.285 |
0.03 |
Yes |
| Sample 20 |
7.577 |
-0.092 |
Yes |
19.139 |
-0.029 |
Yes |
21.263 |
0.008 |
Yes |
| Sample 30 |
7.533 |
-0.136 |
No |
19.194 |
0.026 |
Yes |
21.296 |
0.041 |
Yes |
| Sample 40 |
7.544 |
-0.125 |
No |
19.183 |
0.015 |
Yes |
21.285 |
0.03 |
Yes |
| Sample 50 |
7.522 |
-0.147 |
No |
19.194 |
0.026 |
Yes |
21.285 |
0.03 |
Yes |
| Sample 55 |
7.435 |
-0.234 |
No |
19.172 |
0.004 |
Yes |
21.274 |
0.019 |
Yes |
From Table 2 it is evident that all carbamazepine results and all but one diclofenac results comply with 0.1 min tolerance requirement. But the situation is different with metformin – only two results comply. A study of the metformin retention time differences reveals that all the differences are negative. This indicates that some systematic effect is present. In some cases, the sample matrix affects the retention. Matrix matched calibration could then solve the problem. It is also possible, that the contamination gradually builds up in the column and alters the stationary phase properties and consequently the retention – more extensive clean-up of the sample extracts could help.
In conclusion, retention time tolerance check can be rather restrictive, but it is also helpful for diagnosing possible problems in analytical methods.
Assessment of retention time deviation according to 2021/808.
The validation guideline 2021/808 by the European Commission [] uses the concept of relative retention time (RRT) for establishing the tolerance limit of retention time. The RRT is defined as a ratio of retention time of the analyte (RT) to the retention time of the internal standard (IS). For this example, metformin can be regarded as a retention time internal standard. Experimental data obtained from calibration solutions along with RRT values are presented in Table 3. The averages of RRT-s obtained from calibration solutions serve as reference values for analytes.
Table 3. Absolute (RT) and relative (RRT) experimental retention times obtained from calibration solutions. Metformin is used as an internal standard (IS). Average relative retention times of diclofenac and carbamazepine.
|
Metformin (IS) |
Diclofenac |
Carbamazepine |
|||
|
RT |
RT |
RRT |
RT |
RRT |
|
| Cal 1 |
7.675 |
19.172 |
2.498 |
21.263 |
2.770 |
| Cal 2 |
7.675 |
19.162 |
2.497 |
21.242 |
2.768 |
| Cal 3 |
7.664 |
19.172 |
2.502 |
21.263 |
2.774 |
| Cal 4 |
7.675 |
19.172 |
2.498 |
21.253 |
2.769 |
| Cal 5 |
7.664 |
19.161 |
2.500 |
21.252 |
2.773 |
| Cal 6 |
7.664 |
19.161 |
2.500 |
21.252 |
2.773 |
| Cal 7 |
7.664 |
19.172 |
2.502 |
21.263 |
2.774 |
|
Average (RRTRef): |
2.499 |
2.772 |
|||
Similarly to calibration data, RRT values are now calculated for all analyte peaks in samples (Table 4). To compare RRT values in sample to a reference value, a relative difference (as percent) is calculated as follows:
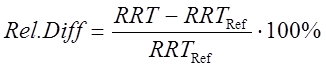 (Eq 1)
(Eq 1)
where RRT is a relative retention time of an analyte in a sample injection and RRTRef is an average of calibration solution RRT-s.
Unsigned value of Rel.Diff is compared to the tolerance limit set by 2021/808: 1%. Results of this assessment are also presented in Table 4.
Table 4. Absolute (RT) and relative (RRT) experimental retention times with respect to the internal standard (IS) in sewage sludge compost extract sample chromatograms. Relative differences (as percentage) of relative retention times from respective reference values are presented along with assessment of the result with respect to the 2021/808 tolerance limit of 1%.
|
Metformin (IS) |
Diclofenac |
Carbamazepine |
|||||||
|
RT |
RT |
RRT |
Rel. Diff. |
Complies? |
RT |
RRT |
Rel. Diff. |
Complies? |
|
| Sample 1 |
7.598 |
19.063 |
2.509 |
0.40% |
Yes |
21.23 |
2.794 |
0.80% |
Yes |
| Sample 10 |
7.566 |
19.183 |
2.535 |
1.40% |
No |
21.285 |
2.813 |
1.50% |
No |
| Sample 20 |
7.577 |
19.139 |
2.526 |
1.10% |
No |
21.263 |
2.806 |
1.20% |
No |
| Sample 30 |
7.533 |
19.194 |
2.548 |
1.90% |
No |
21.296 |
2.827 |
2.00% |
No |
| Sample 40 |
7.544 |
19.183 |
2.543 |
1.70% |
No |
21.285 |
2.821 |
1.80% |
No |
| Sample 50 |
7.522 |
19.194 |
2.552 |
2.10% |
No |
21.285 |
2.83 |
2.10% |
No |
| Sample 55 |
7.435 |
19.172 |
2.579 |
3.20% |
No |
21.274 |
2.861 |
3.20% |
No |
According to Table 4 data, almost all retention times are non-compliant. Only retention times of analytes in the first sample were found to be compliant. A reason for non-compliance is shifting of the retention time of the IS.
Example 2
SANTE criteria for identification and identification points of 2021/808.
Requirements for number and type (molecular, adduct or product ion) of the monitored ions in mass spectrometry are presented in Table 3 of SANTE validation guidelines. For example, the signal from at least two ions must be recorded with any mass spectrometer operated in MS/MS mode regardless of whether mass spectrometer is of low or high resolution. For further requirements see Table 3 in SANTE guidelines [].
The mass spectrometric identification rules used by 2021/808 [] are more elaborate and based on the identification points. The required number of identification points depends on the type of the analyte (see [] for full details): 4 points are required for authorized substances; 5 points are required for unauthorized or prohibited compounds.
Table 5 presents numbers of identification points earned per ion for different techniques.
Table 5. Identification points earned for analysis techniques according to 2021/808.
|
Technique |
Identification points earned |
| Separation (GC, LC etc) |
1 |
| LR-MS ion |
1 |
| Precurson ion (< ±0.5 Da) |
1 (indirect) |
| LR-MSn product ion |
1.5 |
| High resolution MS ion |
1.5 |
| High resolution MSn product ion |
2.5 |
In addition to the data in Table 5, there is one additional rule – each ion may be counted only once. Few examples:
– LC separation, detection with unit resolution MS operated in SIM mode. Three ions recorded – 1+3*1=4 points.
– LC separation, detection with triple quadrupole MS (unit resolution, MS/MS) recording 2 product ions for the same precursor – 5 points (1+1*1+2*1.5=5).
- For example, transitions 142.1→94.1 and 142.1→112.2 for methamidophos (pesticide) analysis.
– LC-MS3 experiment with an ion trap MS (unit resolution) recording 2 product ions – 6.5 points (1+1+1.5+2*1.5=6.5).
– LC separation, detection with combined quadrupole-TOF MS with low resolution precursor ion and 2 high resolution product ions – 6 points (1+1+2*2.5=7.0)
Example 3
Calculation and assessment of ion ratios.
SANTE and 2021/808 limit ion ratio tolerances as follows:
- 2021/808 sets tolerance limit of ± 40% for relative intensities.
- S/N ≥ 3 for all ions.
- SANTE sets tolerance limit of ± 30% for relative intensities.
- S/N ≥ 3 for all ions.
The ion ratio is calculated as an intensity (or peak area) ratio of a less intense ion to that of a more intense ion. The reference ion ratio value is calculated as an average of ion ratios of calibration solutions. Table 6 illustrates the process of calculating such a reference ion ratio on the example of propamocarb (pesticide) calibration.
Table 6. Example of reference ion ratio calculation based on peak areas (S1, S2) of two MRM transitions.
| Propamocarb | 189→102 | 189→144 | Ion ratio |
| S1 | S2 | S2/S1 | |
| Cal. solution 1 |
555 |
259 |
0.47 |
| Cal. solution 2 |
1176 |
499 |
0.42 |
| Cal. solution 3 |
1707 |
803 |
0.47 |
| Cal. solution 4 |
2404 |
991 |
0.41 |
| Cal. solution 5 |
3031 |
1312 |
0.43 |
| Average: |
0.44 |
Evaluated ion ratio is 0.44 (44%). Whether S/N for the transitions is >3, has to be confirmed separately.
In a similar manner, ion ratios are calculated for the analysis of samples (Table 7).
Table 7. Calculation of ion ratio from peak areas (S1, S2) of two MRM transitions recorded from sample chromatograms.
| Propamocarb | 189→102 | 189→144 | Ion ratio |
| S1 | S2 | S2/S1 | |
| Sample solution 1 |
821 |
281 |
0.34 |
| Sample solution 2 |
2221 |
1251 |
0.56 |
To assess the compliance of ion ratios in samples, the following equation can be used:
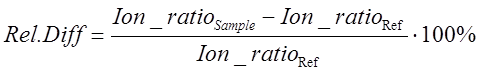 (Eq 2)
(Eq 2)
Relative difference for Sample 1 is -23% and for Sample 2 it is 27%. Comparison with respective limits, reveals that the identity of propamocarb in both samples is confirmed by the rules of both SANTE as well as 2021/808 rules.
Example 4
Selection of product ions.
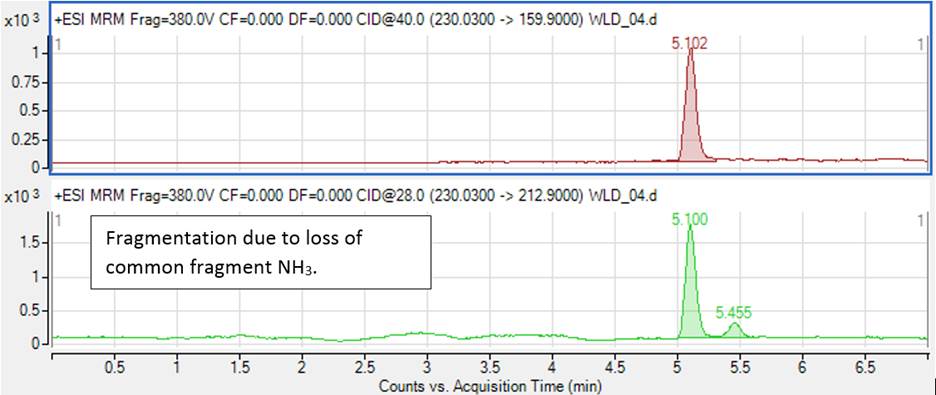
SANTE and 2021/808 give recommendations for a proper selection of the diagnostic ions. In general, fragmentation due to the loss of common fragments (H2O, NH3 etc) should be avoided, as more background noise and interfering compounds are expected. For example, Figure 1 shows transitions 230→213 and 230→160 registered for clonidine (blood pressure lowering drug). Transition 230→213 is due to the loss of NH3, which leads to a noisier background.
Figure 1. Influence of fragmentation pathways on chromatographic background.
Example 5
How well do all the limitations work?
SANTE and 2021/808 set rather restrictive rules to a LC-MS analysis. Do all these criteria guarantee unambiguous confirmation of an analyte? Or maybe the rules are too strict?
A method was developed for a tebufenpyrad (pesticide) analysis in a ginger extract. In Figure 2 overlaid chromatograms of the two transitions of tebufenpyrad are presented for a calibration solution and a ginger extract.
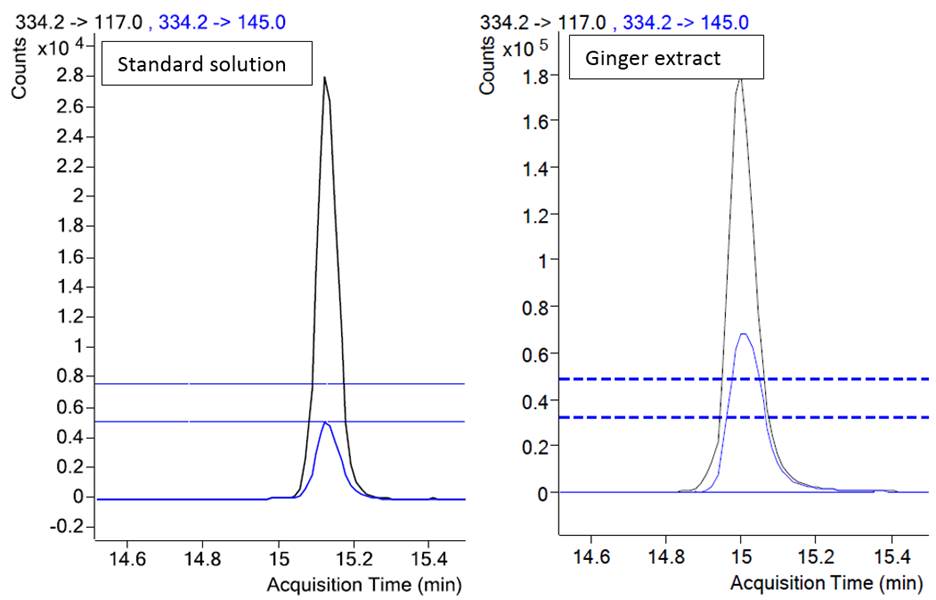
Figure 2. Chromatograms of two transitions of tebufenpyrad in a standard solution and in a sample. (Data from http://www.agilent.com/cs/library/eseminars/public/Triggered%20MRM%20for%20the%20Analysis%20of%20Pesticides.pdf)
Retention times of peaks are similar: 15.1 min in a standard and 15.0 min in an extract and would pass the requirements for the retention time match by SANTE and 2021/808 (0.1 min). The ion ratio of the two transitions for a tebufenpyrad in a calibration standard is 0.21 and for peaks in a ginger extract 0.469. The relative difference of ion ratios is 123%, which exceeds the tolerance limits set by SANTE and 2021/808. Therefore, the presence of a tebufenpyrad in a ginger could not be confirmed. Indeed, further analysis revealed that it was an endogenous ginger compound, which yielded this peak.
So, thanks to the strictness of the rules a result was avoided.