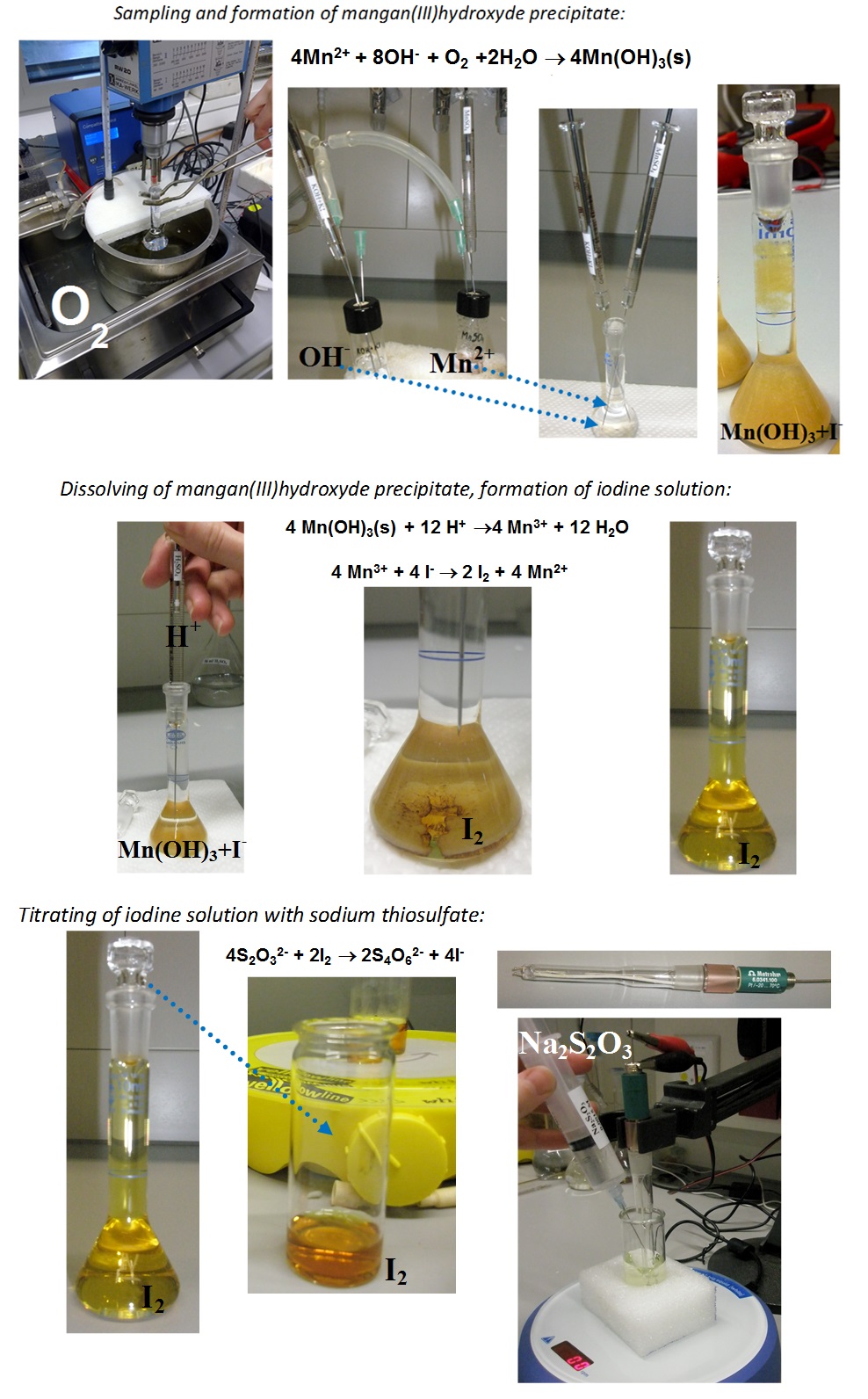
The Winkler method is a titrimetric method for determination of dissolved oxygen (DO) concentration (denoted below as
CO2) in water. For DO determination the sample is taken into the calibrated sample flask that is fully filled with the sample (extra sample is displaced using the stopper. The sample flask volume is denoted as
Vsample. Two reagents, MnSO
4 and alkaline KI solutions (volumes denoted as
VMnSO4 and
VKI_OH, respectively) are added to the bottom of the flask, thereby displacing some of the sample. The following reaction takes place:
4Mn2+ + O2 + 8OH- + 2H2O → 4Mn(OH)3 ↓
All oxygen in the sample is converted to Mn(OH)
3, which precipitates on the bottom of the flask. The solution is then acidified, by adding sulfuric acid (volume is denoted as
VH2SO4). Acid addition must be done carefully, in order to not remove any of the precipitate from the flask, but only the supernatant solution (not containing the analyte).
Mn(OH)3 (s) + 3H+ → Mn3+ + 3H2O
Under acidic conditions Mn
3+ ions oxidize iodide (which was added previously as alkaline KI solution) to iodine, which eventually forms I
3– ions with the excess of I
-:
2Mn3+ + 2I- →2Mn2+ + I2
I2 + I- →I3-
The concentration of the formed I
3– ions is determined by titration with sodium thiosulfate solution. Its volume is denoted in the model as
Vtitrant and concentration is denoted as
Ctitrant (expressed in moles per volume basis):
I3– + 2S2O32– → 3I– + S4O62–
The procedure is described in the picture below:
Which of the following models are correct for finding dissolved oxygen concentration on mass per volume basis (e.g. in mg per litre) in the sample? (
MO2 is molar mass of molecular oxygen)

