
MOOC: Instrumental analysis of cultural heritage objects
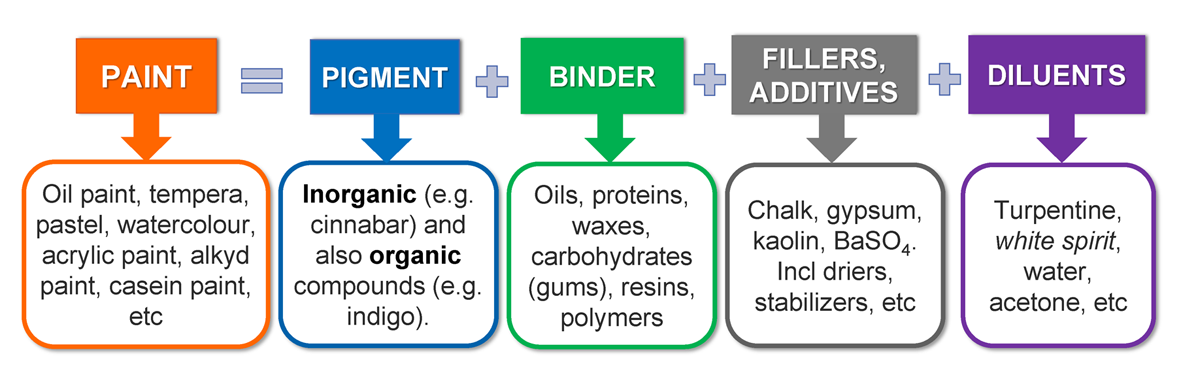
Paints
For centuries, artists have used different painting materials to compose their masterpieces. Paint’s purpose is to decorate and also protect the surface to which it is applied (for example, canvas, wood, metal, paper, stone etc.). The protective role is that of shielding the surface (substrate) from ultraviolet radiation, moisture and oxygen.
Fresh paint is a liquid or semi-liquid mixture composed of colouring matter (pigment) and binder (medium). The pigment is suspended in binder, which forms a strong paint film when it’s dry. Often different fillers, driers or siccatives (for example, salts of cobalt, lead and manganese) and stabilizers (e.g. aluminium stearate, zinc stearate, aluminium palmitate, also beeswax) are added to paint to enhance some properties (e.g. to accelerate drying) of the paint or to make it cheaper. To thin the paint to the best consistency, diluents (solvents) are used. A diluent may be a solvent used to decrease the concentration or viscosity of the paint. It can be added to the mixture only to a limited extent without causing precipitation of the solid pigment [1]. The best-known diluents are turpentine, mineral spirits (or white spirits – hydrocarbon petroleum distillate), different organic solvents (aromatic, esters, ketones, etc.), water [2,3].
There are many different paints categorized by the chemical composition of the binder: oil paints, tempera paints, acrylic paints, alkyd paints, watercolours, etc. (see Fig. 1) [4].
1. Pigments
Pigments are composed of finely divided particles which do not dissolve in binders but are suspended. Pigments typically have high intensity of colour and a high refractive index. Due to these two properties, paints can have intense colour and high hiding power even when the content of the pigment (which is often expensive) is low. Pigments are derived from a wide variety of substances, mostly inorganic but also organic.
Inorganic pigments
Inorganic pigments are natural pigments prepared from minerals (cinnabar, malachite, azurite, etc.), earth deposits, clays (red ochre (also called red earth), yellow ochre, umber, etc.) or are made synthetically [1,3]. Synthetic pigments are made by the processes of chemical synthesis. Some synthetic pigments, like Egyptian blue, lead white, verdigris, have been known since ancient times or earlier.
By their chemical properties, inorganic pigments belong mostly to oxides, sulphides, carbonates, chromates, sulphates, phosphates or silicates of metals [1]. There are few pigments that are complex metallo-organic compounds (like Prussian blue (Fe4[Fe(CN)6]3) or elements in their pure state (such pigments as gold, aluminium and carbon black) [1,4,5].
Inorganic pigments are generally chemically stable and are classified as being among the most stable colouring matters (compared to dyes). However, there are some pigments (for example, cinnabar, red lead, verdigris, etc.) that in paints may show colour changes (dimming, yellowing, browning, darkening) when subjected to intense radiation, such as sunlight or weathering (moisture, air). These processes involve photochemical reactions in which the pigment acts as a catalyst or undergoes chemical changes itself [4,6]. There are also some pigments whose behaviour to strong chemical reagents are different. For example, carbonates, ultramarine, some oxides and sulphides (ZnO, PbO, CdS) are easily decomposed by acids and Prussian blue is sensitive to alkalis. Also, pigments themselves may have either acidic or basic (alkaline) properties. The oxides of heavy metals (ZnO, some of the lead pigments) are basic and they can react with free fatty acids of drying oils to form metallic soaps. This is why lead white [2PbCO3·Pb(OH)2] in oil forms elastic and durable paint film [1,4].
Although the main roles of pigment are colour and hiding power, many of them (e.g. cobalt blue, lead white, red lead, burnt umber, etc.) simultaneously act as siccatives – drying accelerators (driers). Pigments differ widely in their siccative abilities. For example, cobalt blue, umber and lead white are strong siccatives. At the same time, for example ultramarine blue, zinc white, etc., have weak siccative powers.
Organic artists’ pigments
Organic pigments are extracted from plants (e.g. dyer’s madder, indigo, etc.), insects (e.g. cochineal, etc.) or produced by chemical synthesis (e.g. phthalocyanine blue, azo-based pigments like Hansa yellow, permanent red, etc.).
Organic pigments are mostly complex organic compounds that are insoluble in the binding medium, in contrast to dyes that dissolve in the binder to form coloured liquids (see more in the section TEXTILES AND DYES) [4,7]. Often, the same organic compounds (coloured molecules) can be used in dyes as well as pigments. Soluble dyes (for example (dyer’s madder, kermes, etc.) can be converted to insoluble artists’ pigments, for example, by forming insoluble metal salts (lake pigments) from soluble acidic groups present in the dye molecule [7,8] Co-precipitation with some inert, semi-transparent inorganic substance (alum, kaolinite, chalk, etc.) can be used [8]. Although lake pigments can be used with different binding media, they were often used as glazing pigments. Lake pigments were therefore frequently applied in oil or in oil to which a little resin was added to further enhance the translucency.
Development of early synthetic organic pigments started in the 19th century, however until the beginning of the 20th century the use of synthetic organic pigments as artists’ pigments was limited. Synthetic organic pigments can be assigned to pigment groups based on chemical structure (for example, diarylide-, quinacridone- and benzimidazolone pigments) [7].
CLICK HERE to find a table that summarises the most common inorganic and organic pigments for painting, their chemical compositions, when the pigments were taken into use, their refractive indices and some general comments.
2. Fillers
Filler (also called extender) is an inert, colourless or white powdered material used to disperse or dilute pigments [1]. Fillers are used to modify the properties or increase the bulk (volume) of the paint. The aim of adding fillers into paints often is to decrease the quantity of pigments and thereby lower the price of the paint, but also, in some cases these are added to improve some properties of the paint. Fillers are often “inactive” white pigments, which have little or no hiding power or tinting strength when used in a binder. Examples are chalk (CaCO3), gypsum (CaSO4·2H2O) and barium sulphate (BaSO4) [1,3], also some silicates, for example, kaolin (Al2Si2O5(OH)4) and silica (SiO2) [4]. These materials usually have a refractive index below 1.70 in order not to suppress the pigment’s colour too much. Besides being used as fillers, chalk and gypsum are also often used in primer layer – the preparative layer beneath the actual paint.
3. Binders
In its initial state binder is a liquid or semi-liquid substance in which the pigment is suspended. Binder is a material, that upon drying, forms a solid film. Binder provides necessary adhesion and cohesion, keeps the pigment within the coating and ensures that the paint remains attached to the painted surface [2]. Binder can also protect pigment from ambient conditions.
Binders can broadly be divided as follows: oils, proteins, carbohydrates (gums), waxes, resins and synthetic polymers. In the following paragraphs, short descriptions of these materials are given.
Oils
Oils can be divided into drying, semi-drying and non-drying oils. Drying vegetable oils are suitable binders in paints because they form a solid film upon exposure to air [9,10]. The resulting solid film can be very different: matt or shiny, porous or glazed, depending on the quantity and the nature of the pigments or the coating technique [11]. The most important drying oils are linseed oil, poppy-seed oil and walnut oil (CLICK HERE to read more about these oils) [10]. Linseed oil is the most widely used drying oil in paints. Linseed oil dries faster than poppy or walnut oil. Semi-drying oils are, for example, sunflower and soybean oil. Semi-drying oils dry much longer than drying oils and are therefore rarely used in paints [9]. However, they are often added to drying oils to suppress their drying (so that the paint can be used for a longer time) [12]. Non-drying vegetable oils are rice oil, olive oil and castor oil, but these oils are not suitable for painting [1].
Drying oils are natural triacylglycerides (TAGs), also called triglycerides. TAG is a triester in which one glycerol residue is bound to three residues of various fatty acids. An example is presented in Fig. 2. The fatty acid residues are in triglyceride molecules in random combinations. The TAGs in drying oils have a high percentage of C18 unsaturated fatty acid residues, most importantly, linolenic, linoleic and oleic acid. These acids have respectively 3, 2 and 1 double bond(s) in their molecule. The double bonds are responsible for the drying ability of these oils. Fatty acids with more than one double bond are called polyunsaturated fatty acids. Drying oils also contain small amounts of saturated fatty acid residues (mainly palmitic and stearic acid, but in some cases also lauric and myristic acid). [11,13,14]
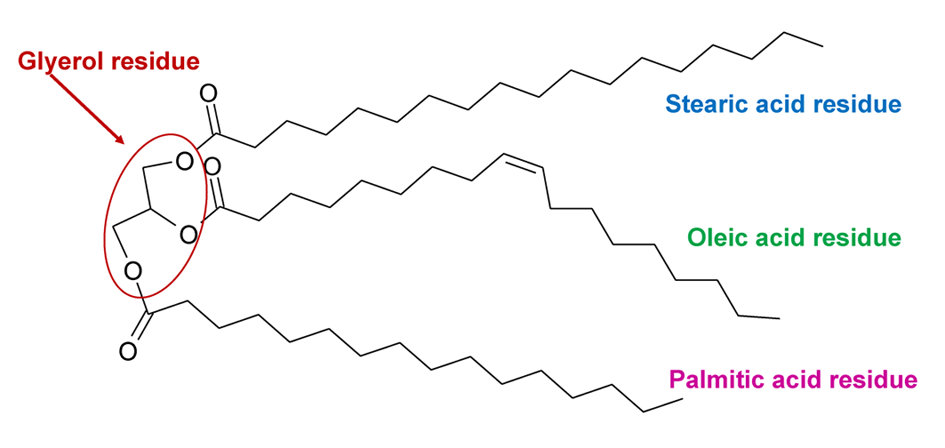
Usually, there are no fatty acid residues characteristic to specific oils, so the oil type can be inferred from the percentages of the fatty acid residues in it (see Table 1). Oils’ drying power is related to the percentage of polyunsaturated fatty acid residues in the oil. Their C=C double bonds enable polymerization and oxidation reactions which promote the formation of the solid film. A description of drying and ageing of linseed oil is presented in the video in the section Chemistry of ageing: III. EXAMPLE: LINSEED OIL.
Table 1. The percentages (%) of fatty acids in triglycerides for the main drying oils [10].
| Fatty acid | Linseed oil | Walnut oil | Poppy oil |
|---|---|---|---|
| Palmitic acid (C16:0) | 4-10 | 9-11 | 3-8 |
| Stearic acid (C18:0) | 2-8 | 1-2 | 0.5-3 |
| Oleic acid (sum of Z+E) (C18:1) | 10-24 | 11-18 | 9-30 |
| Linoleic acid (C18:2) | 12-19 | 69-77 | 57-76 |
| Linolenic acid (C18:3) | 48-60 | 3-5 | 2-16 |
Proteins
Proteins are the most important components of living cells and are found in animal tissues, skin, hair, nails, also hormones, enzymes, etc. Proteins are polymeric substances composed of long chains of various combinations and proportions of twenty naturally occurring amino acid residues [11,15].These amino acid units are linked together by peptide linkage (-CO-NH-). Proteinaceous substances can be found in the composition of binding media (egg white, egg yolk, casein, etc.) in painting materials and animal glue (i.e. hide glue, rabbitskin glue, fish glue, bone glue).
Tempera is a very important painting medium (emulsion) in which pigment is mixed with a water-soluble, typically protein-based, binding agent, such as egg, glue, casein (called egg tempera, glue tempera, casein tempera, respectively) [1,3]. After drying, this binding agent becomes insoluble and permanent. In egg tempera, whole egg (egg yolk and/or white) may be used and some amount of oil, varnish, resins, wax, vinegar, etc., may be added. There are different tempera making recipes available [1,11]. Traditional (used for centuries) egg tempera was made by the simple process of mixing first egg yolk with cold water and then with inorganic pigment paste (the pigment was previously mixed with water into a paste). Vinegar or acetic acid was sometimes added as a preservative to make the medium less greasy [1,4].
By chemical composition, egg yolk is an emulsion which contains about 47% water, ∼33% lipids (triacylglycerides, phospholipids (lecithin), cholesterol), ∼17% proteins (phosvitine, livetine) and ∼3% other compounds (carbohydrates, inorganic substances, etc.) [16].
Egg white (often called also albumen) contains about 88-89% water, 10-11% proteins and a small amount of polysaccharides. Mixture of proteins contains such proteins like ovalbumin (over 50%), conalbumin (∼12%), ovomucoid (∼11%), globulins (∼4%) etc. The dried film from egg white is very brittle and due that plasticizer like glycerol is added [11,16].
Casein is precipitated from skimmed milk by acidification (H2SO4, HCl, etc.). Casein contains a mixture of phosphoproteins (α, β and γ caseins) and in general, casein contains about 1% phosphorus [11,17].
Connective tissue in the skin, bone, hide or muscle of animals (from which animal glue is made) consists mainly of such fibrous protein like collagen. Collagen itself contains repeating sequences of glycine-proline-hydroxylproline and forms a structure of three separate molecules H-bonded and coiled in a α-helical conformation [8,18]. These materials are hydrolyzed and broken down in boiling water, when the solution is cooled jelly-like substance (gel) is obtained, which is gelatine or glue. Gelatine is a purified form of glue.
Carbohydrates (gums, polysaccharides)
Carbohydrates are organic materials composed of natural polysaccharides that typically dissolve or swell in water. The main carbohydrates used as binders are plant gums, honey, starch (also used as adhesive), and cellulose derivatives [15]. Plant gums (e.g. gum arabic) are often used as binders in watercolours (aquarelle) and gouaches.
Plant gums are natural products obtained from different plant species and/or extracted from some specific seeds. They contain complex non-starch polysaccharides with non-crystalline structures that form viscous or gelatinous solutions and do not melt when heated. The main components are different oligo- and polysaccharides, as well as organic acids and their salts with calcium, magnesium or potassium ions. The acids are, first of all, different polyhydroxyacids (combinations of sugar units and acidic units). Sometimes a fatty oil or glycerine is added in small amounts to produce an emulsion that is used for making watercolours. The most important plant gum used as a painting medium is gum arabic (aka acacia gum), which has the highest solubility in water compared to other gums. It is a natural gum consisting of the hardened sap of various species of the acacia tree. Gum arabic has been known since the Egyptian era and has been used for multiple applications (food and pharmaceutical industry, cosmetics, ceramics, polishes, etc.). Its composition is not completely known, since it is a mixture of different oligo- and polysaccharides as well as glycoproteins. Other gums include gum tragacanth, fruit tree gums (e.g. cherry gum) and locust bean gum. [1,19]
Honey has been used as an additive to water-soluble binders (e.g. gum arabic). It mainly consists of monosaccharides (fructose, glucose), disaccharides (sucrose, maltose, etc.) and to a smaller extent of oligosaccharides. Honey also contains proteins, vitamins and smaller amounts of other compounds. Until the 19th century, it was a common additive to moist watercolours to keep them from cracking in dryness. Nowadays, glycerine is used instead in watercolours [1].
Different cellulose derivatives (like cellulose nitrate, methyl cellulose) are used to produce colloidal cellulose coatings that can be dispersed in organic solvents and used as varnishes (or lacquers) and also adhesives. These are light-coloured or white derivatives of cellulose that have several better properties than the starting material. Varnishes made out of these materials are tough, flexible and weatherproof [1].
Waxes
Waxes are stable solid materials that are either of plant or animal origin. They are applied to the object while melted and hot. Waxes consist of various organic compounds, mainly of (1) esters of fatty acids with fatty alcohols, (2) long chain hydrocarbons, (3) free alcohols containing a high number of carbon atoms, and (4) free fatty acids. The process where wax is used as a paint binding medium is called the encaustic technique. However, waxes are used more for the surface coating of paintings. Commonly used waxes are for example beeswax, carnauba wax, candelilla wax, montan wax, paraffin, etc. [1,15].
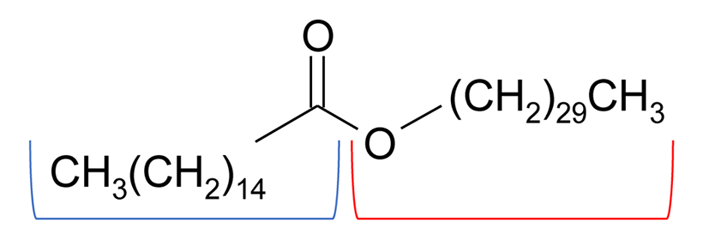
Beeswax has been used since ancient Egyptian times for mummification, as a fixing and adhesive substance and as a paint binder. Recent applications include the production of candles and wax polishes. It mainly consists of myricyl (melissyl) palmitate (C15H31COOC30H61, see Fig. 3), but there are also long-chain alcohols and fatty acids in smaller amounts, as well as other esters. Beeswax is produced by the working bees (apis mellifera) as a by-product in the formation of the cells of the honeycomb and its melting point is about 63°C. The texture, colour and composition may vary greatly depending on the place of production. The colour of beeswax ranges from light yellow to dark brown. It is often bleached to obtain lighter colour [1].
Carnauba wax is obtained from a Brazilian palm, the carnauba tree. After its leaves are dried, the carnauba wax is scraped off and melted in boiling water. It mainly consists of myricyl (melissyl) cerotate (C25H51COOC30H61), but again there are also long chain alcohols, fatty acids and hydrocarbons present in smaller amounts. Carnauba wax is a yellow hard and brittle material that turns light yellow when bleached. It has a high melting point (about 85°C) and is often used in a mixture with other waxes for the coating of paintings [1].
Natural resins
Resins are solid or semi-solid organic materials that can be of natural or synthetic origin. Natural resins are obtained from trees (dammar, mastic, colophony, sandarac, copal, etc.) or insects (shellac) and are usually mixtures of esters, ketones, carboxylic acids, etc., that polymerize over time. Natural resins can form a durable protective layer on the surface. Therefore, they have been extensively used as coating materials, including in the composition of paints. Natural resins alone are not good paint binders because the layer they form on the surface is not elastic enough and tends to darken over time. Therefore, they have been usually used as paint binder additives to achieve some special properties (e.g., to make the paint glossier), as well as varnishes for protecting the paint surface.
More information about resins can be found in the section VARNISHES .
Synthetic resins (polymers)
Synthetic resins (more known as synthetic polymers) are formed by a chemical reaction between two or more substances to form high-molecular materials.The main synthetic polymers used as binders in paints are alkyd resins, acrylic resins (polyacrylates), polyvinyl acetate (PVAc) and also polyvinyl alcohol (PVAL). Synthetic resins are sticky and water-repellent materials that are dissolved in a liquid to produce varnishes or additives for paints (more information in the section VARNISHES). Paints based on synthetic resins have been very popular since their appearance in the late 19th and early 20th centuries thanks to their good durability and ease of use (available as premade paints) [11,17]. Polymer binders also include latex paint, which is obtained by dispersing an aforementioned synthetic polymer together with pigments and additives in aqueous media [20].
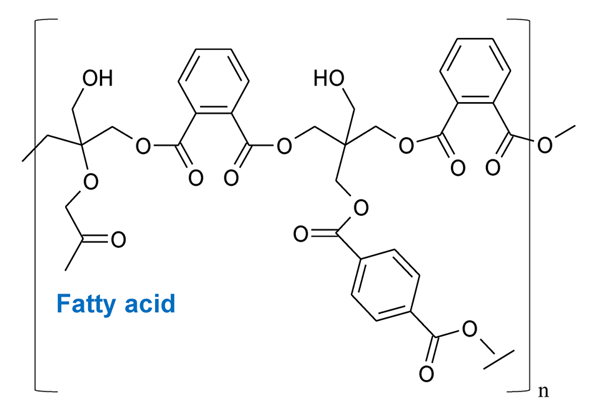
Alkyd resins are the most used synthetic resins in industrial paints and varnishes. They are oil-modified polyesters of a polyhydroxylic alcohol (e.g. glycerol) and dicarboxylic acid (e.g. phthalic acid, used in resin synthesis on the form of anhydride) with various substitutes containing free ‒OH and ‒COOH ends (often fatty acids). An example of the possible structure is given in Fig. 4 [1,21]. Alkyd resins are frequently used with drying oils to produce durable, flexible and not so easily yellowing paints, varnishes and other materials. Alkyd resins are divided into three main groups based on the content of oil: (1) short oil alkyd (oil content below 40 %), (2) medium oil alkyd (oil content 40‒60 %), and (3) long oil alkyd (oil content over 60 %). The oil content is an important factor that affects the properties of the final product. The short oil alkyd is used widely in the production of paints for metal surfaces. The medium oil alkyd is used mainly in the production of enamels and the long oil alkyd dries the longest and is used in the paints of the construction industry. [1,22,23]
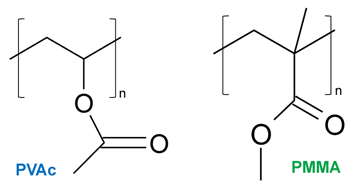
The most widely used vinyl polymers are polyvinyl acetate (PVAc also used PVA), and polyvinyl alcohol (PVAL). PVAc (the structure is shown in Fig. 5) is prepared by the polymerization of vinyl acetate monomer (free radical vinyl polymerization of the monomer vinyl acetate). It is a white, nontoxic thermoplastic resin with very good heat and light stability. It can be produced with different viscosities and can be dissolved in alcohols, ketones, esters, aromatic hydrocarbons and used as an emulsion in water. PVAc is widely used as glue (wood glue, paper adhesive, water-based white glues used for conservation, etc.), as a binder component in paints, inks, etc. Polyvinyl alcohol (PVAL) is usually prepared by hydrolysis of polyvinyl acetate. It is water-soluble and used as a watercolour medium, in papermaking, textile sizing, etc.
Acrylic resins (for instance, PMA ‒ polymethyl acrylate, PMMA ‒ polymethyl methacrylate, aka under trademark Plexiglas) are synthesised by the polymerization of the corresponding monomer (methyl acrylate, methyl methacrylate). The structure of PMMA is shown in Fig. 5. These polymers are known for good heat resistance and stability towards light. For these reasons, acrylic resins are frequently used in coatings and varnishes. [1]
More information about different pigments can be found here:
- Pigment Compendium: A Dictionary and Optical Microscopy of Historical Pigments, New ed.; Eastaugh, N., Walsh, V., Valentine Walsh, Tracey Chaplin, Ruth Siddall, Eds.; Elsevier, Butterworth-Heinemann: Amsterdam, 2008.
- Pigments through the ages: http://www.webexhibits.org/pigments/
- Conservation and Art Materials Encyclopedia Online (CAMEO): http://cameo.mfa.org/
- Gettens, R. J.; Stout, G. L. Painting Materials: A Short Encyclopedia; Dover Publications: New York, 1966.
- Forensic Examination of Glass and Paint: Analysis and Interpretation; Caddy, B., Ed.; Taylor & Francis: London, 2001.
- Seymour, P. The Artist’s Handbook: A Complete Professional Guide to Materials and Techniques; Arcturus, 2003.
- Vahur, S. Expanding the Possibilities of ATR-FT-IR Spectroscopy in Determination of Inorganic Pigments. PhD thesis, University of Tartu, Tartu, Estonia, 2010.
- Pigment Compendium: A Dictionary and Optical Microscopy of Historical Pigments, New ed.; Eastaugh, N., Walsh, V., Valentine Walsh, Tracey Chaplin, Ruth Siddall, Eds.; Elsevier, Butterworth-Heinemann: Amsterdam, 2008.
- Artists’ Pigments: A Handbook of Their History and Characteristics Volume 2; Roy, A., Ed.; Oxford University Press: New York, 1993.
- Kalsbeek, N. Identification of Synthetic Organic Pigments by Characteristic Colour Reactions. Stud. Conserv. 2005, 50 (3), 205–229. https://doi.org/10.2307/25487746.
- Stuart, B. Analytical Techniques in Materials Conservation; John Wiley & Sons: Chichester, England ; Hoboken, NJ, 2007.
- Schramm, H.-P.; Hering, B. Historische Malmaterialien Und Ihre Identifizierung; Akademische Druck- und Verlagsanstalt: Graz, 1988.
- van den Berg, J. D. J. Analytical Chemical Studies on Traditional Linseed Oil Paints; FOM-Institute for Atomic and Molecular Physics (AMOLF): Amsterdam, The Netherlands, 2002.
- Masschelein-Kleiner, L. Ancient Binding Media, Varnishes and Adhesives; ICCROM technical notes series; ICCROM: Rome, 1995.
- Blaško, J.; Kubinec, R.; Husová, B.; Přikryl, P.; Pacáková, V.; Štulík, K.; Hradilová, J. Gas Chromatography/Mass Spectrometry of Oils and Oil Binders in Paintings. J. Sep. Sci. 2008, 31 (6–7), 1067–1073. https://doi.org/10.1002/jssc.200700449.
- Mallégol, J.; Lemaire, J.; Gardette, J.-L. Drier Influence on the Curing of Linseed Oil. Prog. Org. Coat. 2000, 39 (2–4), 107–113. https://doi.org/10.1016/S0300-9440(00)00126-0.
- Lazzari, M.; Chiantore, O. Drying and Oxidative Degradation of Linseed Oil. Polym. Degrad. Stab. 1999, 65 (2), 303–313. https://doi.org/10.1016/S0141-3910(99)00020-8.
- Derrick, M. R.; Stulik, D.; Landry, J. M. Infrared Spectroscopy in Conservation Science; Scientific tools for conservation; Getty Conservation Institute: Los Angeles, 1999.
- Phenix, A. The Composition and Chemistry of Eggs and Egg Tempera. In Early Italian paintings: techniques and analysis ; symposium [… organized by the Stichting Restauratie Atelier Limburg on 9 – 10 October 1996 at The Bonnefantenmuseum Maastricht]; Bakkenist, T., Hoppenbrouwers, R., Dubois, H., Stichting Restauratie Atelier Limburg, Bonnefantenmuseum, Eds.; Limburg Conservation Institute: Limburg, 1997.
- Mills, J. S.; White, R. The Organic Chemistry of Museum Objects, 2. ed., reprinted.; Butterworth-Heinemann series in conservation and museology; Butterworth-Heinemann: Oxford, 2003.
- Horie, C. V. Materials for Conservation: Organic Consolidants, Adhesives and Coatings, Reprinted.; Butterworth-Heinemann series in conservation and museology; Butterworth-Heinemann: Oxford, 2005.
- Granzotto, C. Methodological Developments Based on Mass Spectometry for the Analysis of Glycoproteins and Polysaccharides of Plant Gums: An Application to Cultural Heritage Samples; Ca’ Foscari University of Venice: Venice, Italy, 2014.
- Johnson, R. L.; Robertson, D. N. Film Forming Aqueous Colloidal Dispersions Containing Aromatic Nitroalkanols and Method for Preparing Same, 1957.
- Crowl, V. T.; Malati, M. A. Adsorption of Polymers and the Stability of Pigment Dispersions. Discuss. Faraday Soc. 1966, 301–312.
- Alkyd resin | chemical compound. Encyclopedia Britannica. https://www.britannica.com/science/alkyd-resin (accessed 2018-10-31).
- Polynt – Alkyd Resins for Paints. Http://Www.Polynt.Com/En/Alkyd-Resins-for-Paints/.


