
MOOC: Instrumental analysis of cultural heritage objects
Varnishes
Varnishes are composed of resin(s) dissolved in a liquid, such as a linseed oil, turpentine, spirit, etc. [1]. Different additives, such as mattifying and drying agents (such as wax, etc.) can be added in order to improve the quality/properties of the varnish. Upon drying, the varnish forms a hard and transparent film that protects the object from humidity, sunlight and dust, also from smaller physical and biological damages. In addition to protecting the objects (paintings, also furniture, musical instruments, etc.), varnishes have an aesthetic role: they help to accentuate and brighten the colours on the paintings, highlight the structure of the wood, etc.
The main component in varnishes is resin (or resins). Resins are highly viscous or solid materials of natural or synthetic origin that contain pre-polymer compounds with reactive groups [2]. They are sticky and water-repellent materials with good adhesive and film-forming properties [3,4]. Resins from different sources differ in their composition (see Fig. 1).
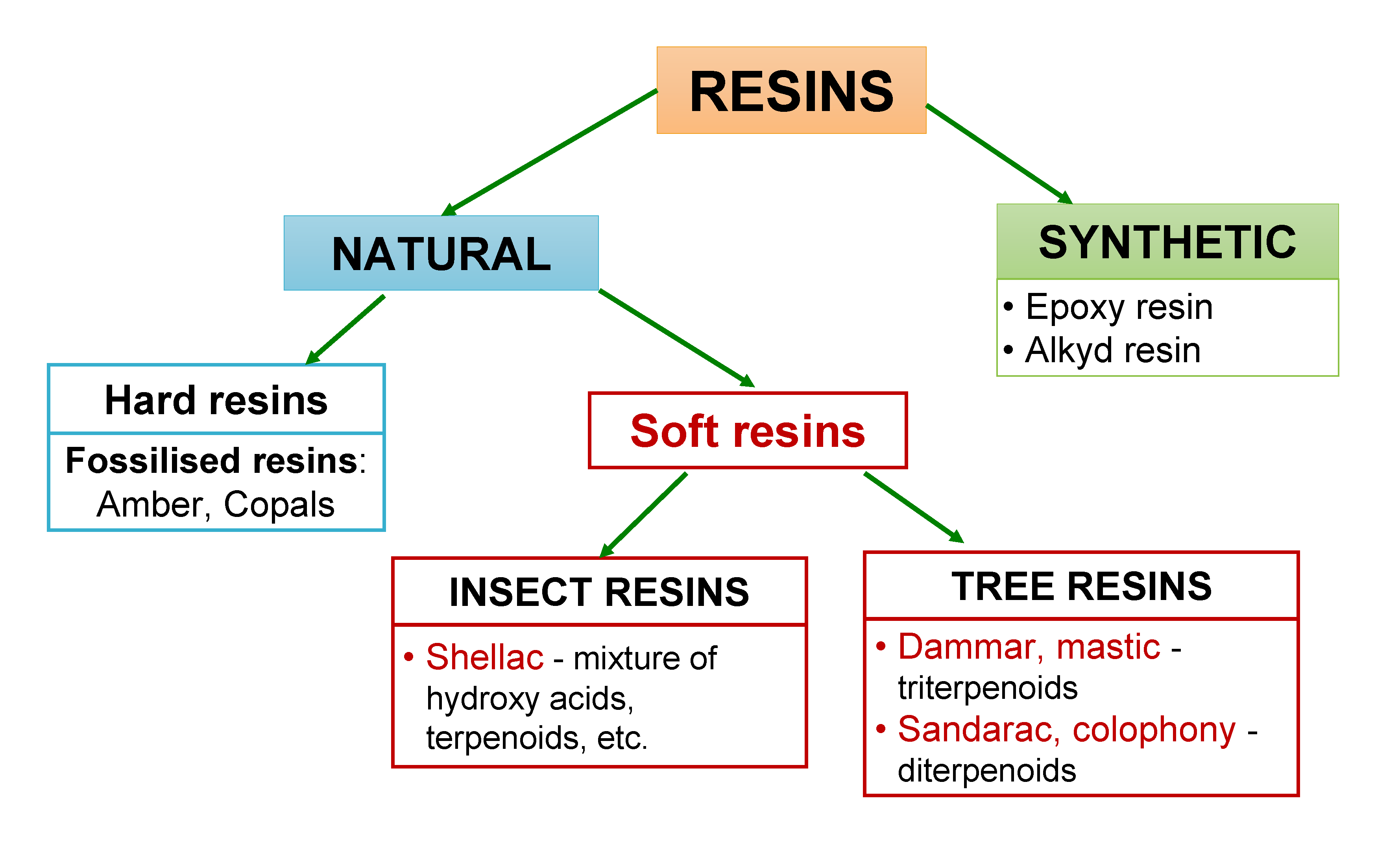
1. Natural resins
Natural resins are by-products secreted by plants, notably trees, and also by some insects [1,3]. In their raw and non-purified form, they consist of numerous organic compounds, such as terpenoids and terpenes, alcohols, esters, essential oils, phenolic compounds, water, etc. [1,4].
Tree resins
Tree (plant) resins can be divided into terpenoid and phenolic resins. The majority of tree resins are composed of terpenoids (also, some amount of terpenes). Terpenoids are chemically altered (oxidized, dehydrogenated, etc.) terpenes. The “building blocks” of terpenes are isoprene (C5H8) units. Examples of structures of different terpenes and terpenoids found in resins are presented in Fig. 2.
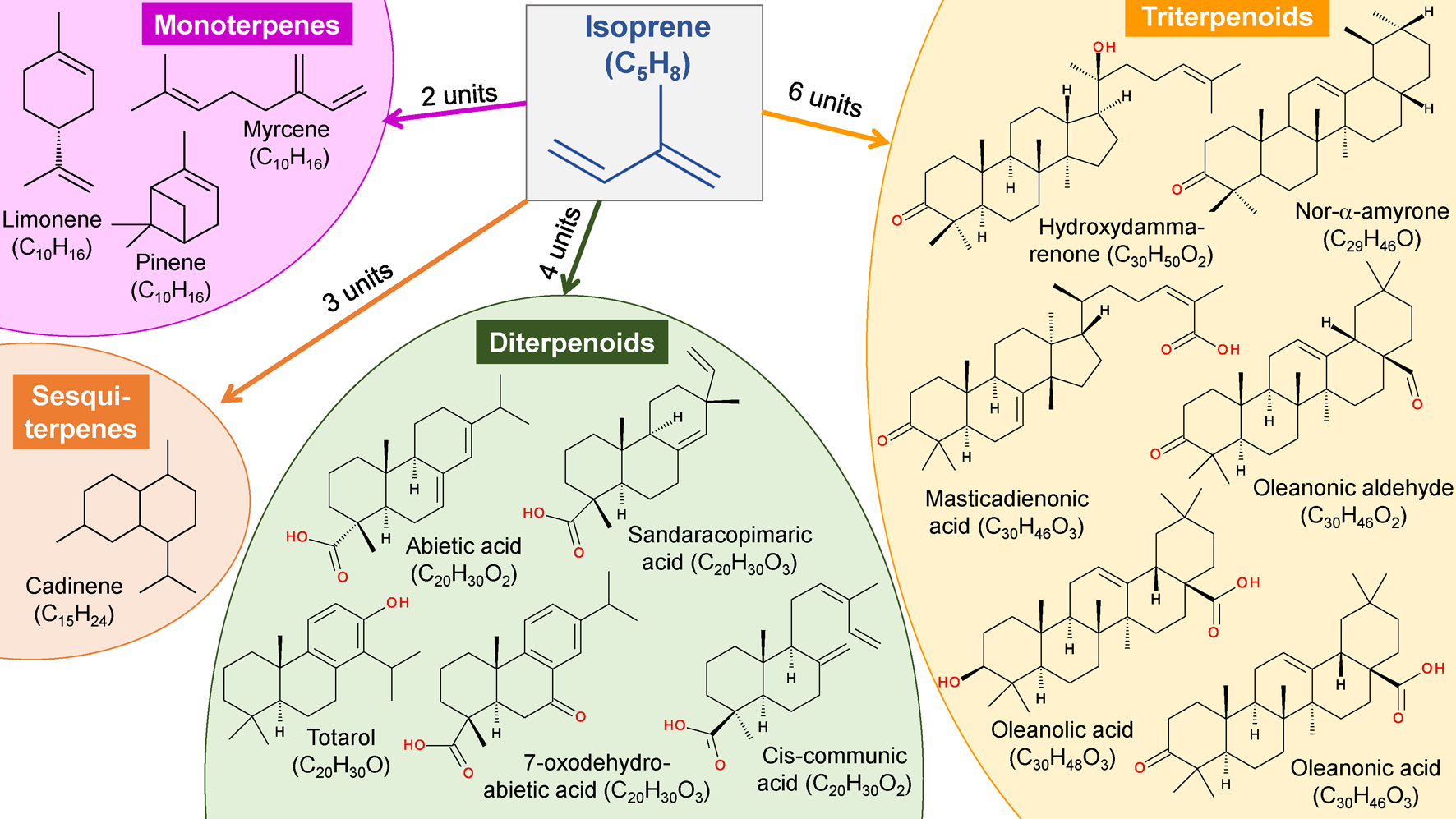
Tree resins usually contain di- or triterpenoids (containing 4 or 6 isoprene units, respectively) along with mono- and sesquiterpenoids (containing 2 and 3 isoprene units, respectively). Di- and triterpenoids are almost never found together in the same resin. Therefore, the tree resins can be divided into di- and triterpenoid resins [1,4]. There are several tri- and diterpenoid resins. The most common triterpenoid resins are dammar and mastic resin (CLICK HERE to read more about these resins).
Sandarac and colophony resin (CLICK HERE to read more about these resins) belong to diterpenoid resins. All of these resins have been used to produce varnishes for different types of objects and purposes – picture, retouching, musical instruments, furniture, etc.
Phenolic resins (known also as balsamic resins) are composed of aromatic compounds, mainly cinnamic and benzoic acids and their esters. Phenolic resins, such as benzoe resin and storax resin have found use in medicinal applications and in the production of cosmetics [1,4].
Insect resins
In addition to trees and plants, also, some insects produce resinous material during their life time, usually for their protection. The most important resin of animal origin is shellac resin (CLICK HERE to read more about this resin) that has been extensively used for the production of furniture varnish.
2. Synthetic resins
Within the last century a number of synthetic materials have been incorporated into art and conservation, among others several varnishes based on synthetic resins.
Synthetic resins are solid or semi-solid amorphous high molecular weight materials that are produced during polymerization or polycondensation reactions [1]. By their physical properties synthetic resins generally resemble the natural resins: they are hard to semi-hard water-insoluble materials, that are soluble in organic solvents. From the chemical aspect, they are very different. Synthetic resins are obtained by polymerization and/or condensation of monomers. Different compounds are used as monomers, depending on whether the resin is obtained via polymerization or polycondensation. In the case of polymerization, they are molecules with double bonds (often acrylic acid derivatives). In the case of polycondensation, they are organic molecules with at least two functional groups (e.g. -OH, -COOH, acid anhydride, etc.). Synthetic resins (polymers in general) can have linear or branched structures. The structure is determined by the number of functional groups in the monomers. If the monomers have more than two functional groups, then branched and/or cross-linked products are formed.
One might assume that the chemistry of synthetic resins, and thus, varnishes are better known than that of natural resins. This is only partially so, however, because the composition of the majority of synthetic varnishes is proprietary information of the manufacturers, meaning the actual components of the varnish may not be publicly revealed. So, similarly to the situation with natural resins, the knowledge about the composition, properties and ageing effects of synthetic resins and varnishes comes through scientific studies.
Two main types of synthetic resins are vinyl polymers and condensation polymers.
- Vinyl polymers are formed during the polymerization of double bond-containing monomers. There are several vinyl resins that have been incorporated as art and conservation materials, such as polyacrylates (Paraloid B-72, Paraloid B-67, etc.), polyvinyl alcohol (PVAL), polyvinyl acetate (PVA or PVAc), and polyvinyl chloride (PVC), etc.
- Condensation polymers, as the name implies, are formed during the condensation reaction of monomers. Among condensation, polymers are alkyd resins (mainly used as paint medium, also suitable for producing varnish for woodwork), ketone resins, epoxy resins (excellent adhesives), etc.
More information about synthetic resins can be found in the PAINTS section.
- Mills, J.; White, R. Organic Chemistry of Museum Objects, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2003.
- McNaught, A. D.; Wilkinson, A. IUPAC. Compendium of Chemical Terminology, 2nd ed. (the “Gold Book”); Blackwell Scientific Publications: Oxford, UK, 1997.
- Langenheim, J. H. Plant Resins: Chemistry, Evolution, Ecology, and Ethnobotany; Timber Press, Inc.: Portland, USA, Cambridge, UK, 2003.
- Colombini, M. P.; Modugno, F. Organic Mass Spectrometry in Art and Archaeology; John Wiley & Sons, Ltd.: Chichester, UK, 2009.


