
MOOC: Instrumental analysis of cultural heritage objects
Textiles and dyes
Identification of textile fibres and dyes is essential to learn more about historical artefacts: knowledge of the composition can provide information about the age, origin and condition of the textile piece. In order to select suitable techniques for the analysis of real-life textiles and dyes, it is important to know the chemical and physical properties of the different types of fibres and dyes. Below is a brief overview of the properties of the most common textiles and dyes.
1. Textile fibres
Textiles are flexible woven materials. Structurally they are complexes of chaotic or directionally elongated molecules [1]. Textiles consist of fibrous materials, which by their chemical composition are different kinds of polymers. By their origin, textile fibres can be classified as natural and man-made fibres. Natural fibres can originate from plants or animals. Man-made fibres can be regenerated from natural sources (for example, cellulose) or synthezised.
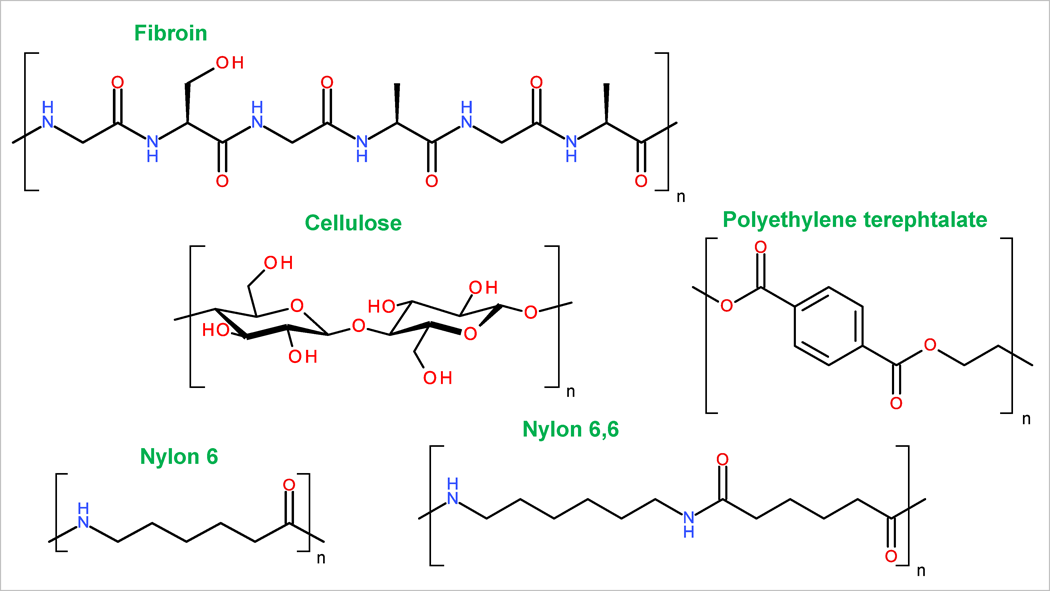
Natural fibres
The most common plant fibres are cotton and linen, however also hemp and jute have been used. Plant (or vegetable) fibres are mainly cellulose-based, and their chemical composition is very similar. In addition to cellulose, they contain small amounts of hemicellulose, fatty substances, pectins, mineral fragments and water. Cellulose is a linear polymer of glucose, which in its simplest form is β-1,4-linked units (see Fig. 1), however hemicellulose is a more complex group of non-linear polysaccharides. The ratio of cellulose and hemicellulose depends strongly on the fibre type. For example, while cotton is almost entirely cellulose-based, however linen can contain significant amounts of hemicellulose.
The most used animal fibres are wool and silk, which are proteinaceous materials. Wool can be obtained from many animals, such as alpaca, rabbit, yak, goat (e.g. cashmere), etc., but sheep wool is most commonly used. Wool contains mainly keratin (α-keratin) that in most parts consists of residues of alanine, arginine, leucine, and cysteine. Silk is a highly valuable fibre due to its strength, elasticity, softness, durability and ability to bind chemical dyes. Although silk fibre is obtained from several insects, commercially only filament produced from silk moth Bombyx mori is used for textile making. Pure silk contains around 70–80% of fibroin and 20–30% of sericin. Sericin is the glue that is dissolved during processing and, thus, the final silk textile consists mostly of fibroin (see Fig. 1). Fibroin consist mostly of glycine, alanine, serine and small amounts of cysteine residues.
Regenerated fibres
There are also different regenerated (modified) fibres like acetate, viscose and lyocell. [1–3] These fibres are produced from cellulose using different solvents and chemicals. Viscose fibre is produced (firstly in 1898) from wood and is chemically hydrocellulose, while in acetate fibre (first man-made thermoplastic fibre), at least 74% of hydroxyl groups in cellulose are acetylated. Lyocell has been produced since 1980s, and is made by dissolving cellulose in an organic solvent (N-methyl-morpholine N-oxide), after which fibres are spun by extrusion to a spinning bath. Lyocell is mainly sold by its trademark TencelTM.
Synthetic fibres
Synthetic fibres are made from high molecular weight compounds that are produced from coal, petroleum or natural gas. Chemical structures of synthetic fibres are highly diverse. Polyamide (the first synthetic fibre) was synthesized for the first time in the 1930s. Polyamide, also called nylon, is a general name for aliphatic polyamides, the most common ones being nylon 6 and nylon 6,6 (see Fig. 1). Polyester (started to synthesize in the 1940s) is chemically polyethylene-terephthalate (see Fig. 1). The composition of polyacrylic (polyacrylonitrile) fibre is more complex. It is a co-polymer consisting of at least 50% of the acrylonitrile monomers, the rest being acrylic acid esters, vinyl acetate, acrylamide and methacrylic acid ester monomers in different ratios. [1–3] Elastane (also known as spandex), nowadays widely used highly elastic fibre, is a block copolymer in which rigid segments with urethane and urea linkages alternate with elastic polyether segments. This structure gives elastane its unique elasticity.
2. Textile dyes
Textile dyes are different organic molecules that give intense colour in small quantities and can bind strongly to textile fibres. Dyes can be classified by their origin (natural, synthetic), chemical composition (anthraquinones, flavonoids, indigoid, azo, etc.) or dyeing method (direct, mordant, vat). [4]
The oldest known written evidence of usage of textile dyes dates back to 6000 AD, but since fibres are very fragile, no physical proof of such old dyed textiles has preserved. According to ancient texts, in Egypt, in the first century, indigo was used for obtaining blue, kermes for red, and sea-buckthorn berries for yellow textiles. [5] Until the 19th century, only natural dyes were used, but since then many synthetic dyes have been developed. Both natural and synthetic dye components can be very different by their chemical composition and structure, their binding features and stability [5].
Different textile fibres bind dyes differently. According to the dyeing techniques dyes are divided into three groups: direct dyes, mordant dyes, and vat dyes [4].
Direct dyeing involves just soaking and boiling dye sources and textile fibre in order to attach the dye components into the fibre.
The most common dyeing approach with natural dyes is mordant dyeing, where dyeing is done with the help of mordants. Tannins, alum (KAl(SO4)2·12H2O), tin(II)chloride, potassium dichromate, urine etc., are examples of substances used as mordants. When dyeing with mordant, fibres can be processed with the mordant solution before dyeing, during dyeing or in some cases after dyeing. The exact mechanism of action of mordants is in many cases still not entirely understood, but in principle, mordant helps dyes better attach to the fibre. In the case of mordants based on metal salts, complex systems can occur where metal cation simultaneously forms bonds with functional groups in fibre and with functional groups of the dye molecule, thereby chemically linking the dye to the textile.
Vat dyeing is a technique based on the chemical reactivity of the dye. Dyeing is initially done with a soluble form of the dye. Then it is exposed to oxygen, and as a result, the insoluble dye form is obtained that will be firmly attached to the fibre. The best-known vat dye is indigo, where soluble precursors from plants (indican, isatan B) are going through oxidation to form insoluble indigo dye. [4,5]
Natural dyes
The range of natural dyes is very wide. People have used plants, insects, mushrooms and lichens to obtain many different colours and shades.
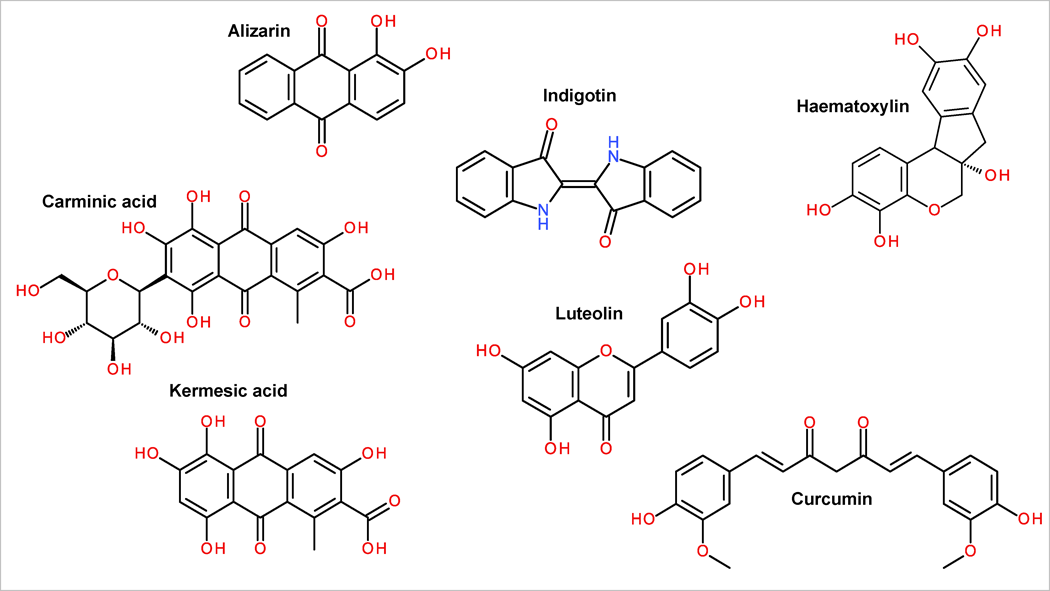
The most common red dyes are different anthraquinone based dyes like dyer’s madder (Rubia tinctorum L.) with the main component alizarin (see Fig. 2.), but also containing purpurin, lucidin, etc. Other dye sources in the Rubiaceae family are wild madder (Rubia peregrina L.), Indian madder (Rubia cordifolia L.), northern bedstraw (Galium boreale L.) and other similar plants – all giving red colours in different shades and intensities. Anthraquinone dyes are also present in popular dyeing sources like cochineal insect (Dactylopius coccus) where the main component is carminic acid (see Fig. 2), and dyer’s kermes (Kermes vermilio) with main components of kermesic acid (see Fig. 2) and flavokermesic acid. Besides anthraquinone dyes, there are other sources for obtaining red dyes. Red and pink colours can be extracted from florets of safflower (Carthamus tinctorius L.) where the main source for red is carthamin. For getting red coloured textiles with this plant, it is important to wash out all the yellow dye components from the petals prior to dyeing process. Red colour can also be obtained from brazilwood (Caesalpinia echinata), red sandalwood (Pterocarpus santalinus L.).
For yellow colour, flavonoids have probably the most important place among the dyes. One of the most common example is dyeing plant weld (Reseda luteola L.), where the main source for yellow colour comes from flavone named luteolin (see Fig. 2). Weld has been used to dye different natural fibres like silk, wool, cotton and linen, each of them needing mordanting with alum or alum and cream of tartar (potassium bitartrate). As luteolin has good light resistance, several other dye sources that also contain this component have been used for dyeing. Some of the examples being saw-wort (Serratula tinctorial L.) and dyer’s broom (Genista tinctorial L.). Other flavonoids like rhamnetin, quercetin and kaempferol are important dyeing components in dyer’s buckthorn (Rhamnus saxatilis) and fisetin in young fustic (Cotinus coggygria). Besides flavonoids, yellow colour can come from carotenoids obtained from saffron (Crocus sativus L.), cape jasmine (Gardenia augusta L.), etc. But the most popular yellow dye (mostly for food colouring) in the world is turmeric (Curcuma longa) with a collection of different curcuminoids like curcumin, demethoxycurcumin and bisdemethoxycurcumin (see Fig. 2).
For obtaining blue colour from natural sources, indigo plants are the most important. As indigo dye is a vat dye, a special dyeing process is used for getting insoluble and permanent indigotin (see Fig. 2) to the fibre. In plants, indigo dye is stored as several indigo precursor molecules. In Indian/common indigo (Indigofera tinctorial L.) the main component is indican, while in woad (Isatis tinctorial L.) the main precursors are isatan A and isatan B. There are however, many other indigo plants all over the world with these precursors, all giving finally the indigo colour with insoluble blue indigotin. As by-products, different indigotin isomers like indirubin, isoindigotin, etc. can occur.
Logwood (Haematoxylum campechianum L.) with its main component haematoxylin (Fig. 2) has an amazing ability to give an extensive range of different colours depending on the mordant used. Unfortunately, most of the shades are not very colourfast and thus only black colours (obtained with ferrous sulphate mordant) are the ones that retain their colour for longer. For example, with the help of alum and cream of tartar navy blue is obtained for wool, while tin and cream of tartar as mordant give purple tones. For getting other darker shades (browns and black), different tannin-containing plants are used. Tannins are polyphenols that are also used in many cases as mordants. Brown and black colours can be gained by using galls from different oaks like pedunculate oak (Quercus robur L.), durmast oak (Quercus petraea L.), gall oak (Quercus infectoria L.), as well as other trees.
Synthetic dyes
The first synthetic dye was mauveine, synthesized by William Henry Perkin in 1856. After that, a row of different synthetic dyes has been developed, and by now, synthetic dyes have taken over the dyeing world. A significant advantage of synthetic dyes is their good resistance to light, oxidation and washing. Another advantage is the wider range of colours and shades available compared to natural dyes. Moreover with synthetic dyes, very different and bright colours can be obtained that are not found in nature. In principle, synthetic dyes can be prepared according to the specific needs – what colour and on what type of textile is needed. The structure of the synthetic dyes can be modified by adding functional groups that help to attach better to the fibre (e.g. cyclic imide system in direct dyes) or increase solubility (sulphonic acid group).
Synthetic dyes are used to give colour not only to textiles but to food, plastics and other consumer goods. All commercial synthetic dyes have Colour Index (C.I) Name and Number (e.g. C.I Acid Red 37, C.I Acid Orange 7).
By their chemical composition, synthetic dyes can be divided into 25 classes. The most important synthetic dyes are azo, anthraquinone, and phthalocyanine dyes.
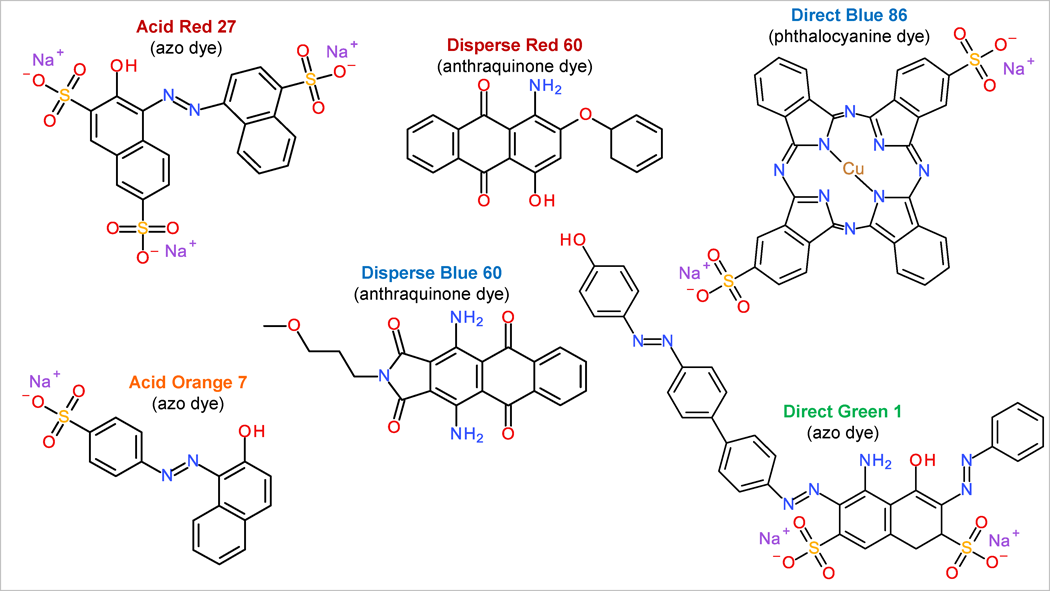
The most famous synthetic dye group is probably azo dyes – approximately 66% of all the colorants are azo dyes. Azo dyes contain N=N bond in the chemical structure (examples shown in Fig. 3). Another important dye group is anthraquinone dyes. In this chemical group there are also several important natural compounds like alizarin, purpurin, etc.; two noticeable mentions in synthetic dye field are C.I Disperse Red 60 and C.I Disperse Blue 60 (Fig. 3).
Phthalocyanines are formed by linking four isoindole fragments with nitrogen bridges and coordinated with metal atoms. Most important phthalocyanine dyes are different copper phthalocyanides that are used for their brilliant blue and green colours, e.g. C.I Direct Blue 86 (Fig. 3).
For actual dyeing in practice, much more important than the chemical grouping is grouping by the application method. For required colour, usually, mixtures of dyes are used and thus, it is important to know that different dyes and fibres are compatible. However, this is a much broader topic and is not discussed here in detail.
Further reading:
- Good overview of various dye sources: Cardon, D. Natural Dyes: Sources, Tradition, Technology and Science; Archetype: London, 2007.
- More about synthetic dyes and their properties: Mahapatra, N. N. Textile Dyes; Woodhead Publishing India Pvt. Ltd: Daryaganj, New Delhi, 2016.
- Identification of Textile Fibers, 1st edition.; Houck, M. M., Ed.; Woodhead Publishing: Boca Raton, 2009.
- Hearle, J. W. S.; Morton, W. E. Physical Properties of Textile Fibres, Fourth Edition, 4th edition.; Woodhead Publishing: Boca Raton, Fla., 2008.
- Peets, P.; Leito, I.; Pelt, J.; Vahur, S. Identification and Classification of Textile Fibres Using ATR-FT-IR Spectroscopy with Chemometric Methods. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2017, 173, 175–181. https://doi.org/10.1016/j.saa.2016.09.007.
- Cardon, D. Natural Dyes: Sources, Tradition, Technology and Science; Archetype: London, 2007.
- Delamare, F.; Guineau, B. Colour: Making and Using Dyes and Pigments; Thames & Hudson Ltd: London, 2000.
- Mahapatra, N. N. Textile Dyes; Woodhead Publishing India Pvt. Ltd: Daryaganj, New Delhi, 2016.


