
MOOC: Instrumental analysis of cultural heritage objects
5.2. Mass spectrometry
1. General aspects of mass spectrometry
Mass spectrometry (MS) is a highly selective and sensitive analytical technique that is used for the qualitative and quantitative analysis of a wide variety of materials. In MS, sample compounds are converted into gas-phase ions and their mass-to-charge (m/z) ratios of those ions are measured. Mass spectrometry can be used as a standalone technique (direct MS) or combined with chromatographic techniques (GC-MS, LC-MS).[1]
In the MS instrument, the sample molecules are ionised in the ion source (via strong electric fields or by bombarding the molecules with electrons, photons, or other molecules/ions) and guided to the mass analyser, where the ions are sorted according to their m/z ratios. The sorting of the ions requires high vacuum conditions: the pressure is in the range of 10-5 … 10-10 bar, depending on the instrument type. Vacuum is required to minimise the risk of losing the created ions as a result of collisions with air molecules. The high vacuum is achieved with a series of vacuum pumps. After “sorting” ions in the mass analyser, the separated ion packages are detected by the detector. The collected data is presented as a mass spectrum – a plot of ion abundance vs m/z ratio. In Fig 1. a schematic drawing of MS instrument is presented. [1,2]
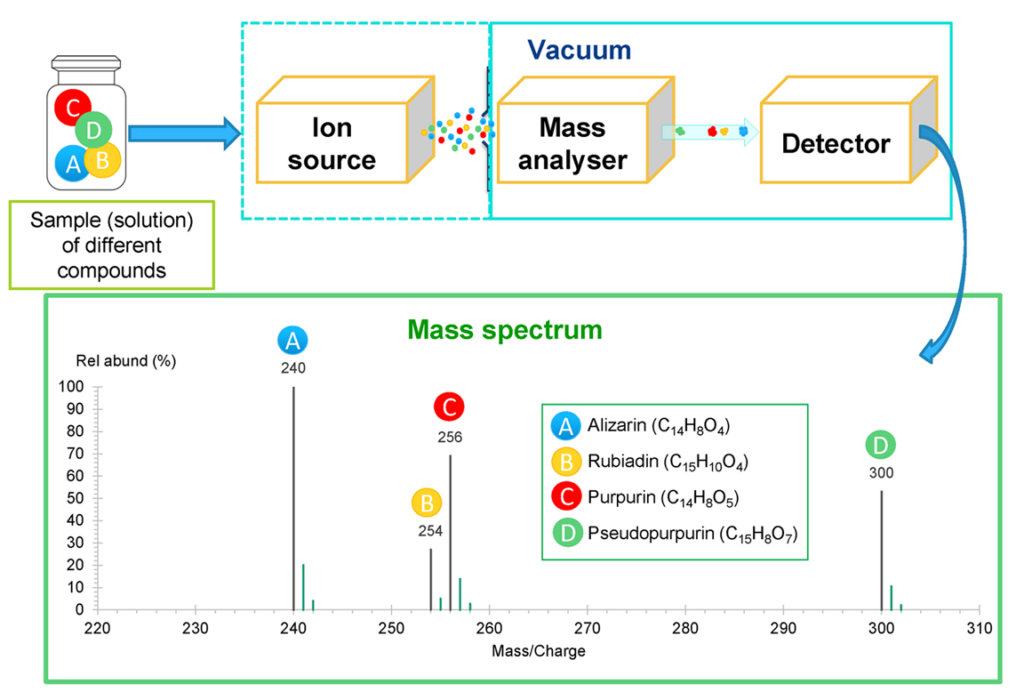
Ion sources
The selection of different ion sources is extensive. Ion sources can be divided into soft and hard. In the case of hard ionisation, the energy transferred to the analysed molecules during ionisation is high. This causes extensive fragmentation of the formed molecular ions M+ – ions formed via removing an electron from the initial molecule M. The molecular ions are chopped into smaller fragments during ionisation. This leads to a complex mass spectrum with several peaks that contains structural information about the sample components. The most common hard ionisation technique is electron ionisation (EI), which is widely used in GC-MS systems. [1,2]
The majority of the ion sources are soft ion sources. This means that the energy transferred to the sample molecules M during the ionisation is rather low and therefore, the formed ions do not fragment extensively. These ion sources can produce either positive or negative ions, depending on the source polarity chosen by the operator. Ions are formed mostly by protonation [M+H]+, cation (e.g., Na+) addition [M+Na]+ or by deprotonation [M-H]–. These ions are called quasimolecular ions (protonated/deprotonated) and adduct ions (addition of a cation) and are the ones typically observed in mass spectra (typically no fragmentation occus). Examples of soft ionisation methods are electrospray ionisation (ESI), atmospheric pressure chemical ionisation (APCI), chemical ionisation (CI), and matrix-assisted laser desorption/ionisation (MALDI). The applicability of ion sources depends first of all on the sample phase – gas, liquid or solid. The suitability of an ion source depends on the sample (molecule mass, polarity, solubility, volatility, etc.) and the type of information required.
Commonly used ion sources [1,2]:
- Electron ionisation (EI) is mostly used with GC-MS. Sample molecules (M) in gas phase are ionised by bombarding with high-energy electrons and at first molecular ions M+ are formed. These ions then fragment into smaller ions. EI operates in a vacuum. Sample can be introduced as liquid, gas or solid (needs heating) in the EI source. EI can ionise virtually all compounds, has good ionisation efficiency, good sensitivity and the fragmentation pattern gives information about the structure of the compounds. However, in case of extensive fragmentation, the molecular ion may not be observed, which complicates identification. Also, the EI mass spectrum is complex and for interpretation, reference mass spectra (library) are often needed. With EI, different oils, resins, hydrocarbons, aromatic compounds etc., can be analysed.
- Chemical ionisation (CI) is also mostly used with GC-MS. Sample compounds in gas-phase are ionised by the reaction with gas-phase ions (e.g. protonated methane CH5+). Mostly [M+H]+ ions are formed. CI operates in a vacuum. Little if any fragmentation occurs in CI, and thus, the mass spectrum is much simpler than in the case of EI. Also, a limited amount of structural information is obtained. With CI, lipids, amino acids, carbohydrates, etc., can be analysed.
- Electrospray ionisation (ESI) is compatible with LC-MS and can also be used with direct MS. A sample solution is sprayed into the electric field in ion source where the charged droplets are created. The solvent is gradually evaporated until gas-phase ions, typically [M+H]+ or [M-H]–, are formed. This source operates at atmospheric pressure. With ESI, preferably more polar compounds, especially those that have basic or acidic properties, are ionised. The sample must be completely soluble in a volatile solvent or a volatile solvent mixture. It is a very soft and sensitive ionisation method and is suitable for determining compounds at very low concentrations. Compounds with very high molecular mass (approx. 100000 Da) can be analysed. The disadvantages of ESI are that the sample must be fully dissolved – only soluble part of the sample can be analysed (which is an advantage in the case of LC), reproducibility is not always good, and compounds can suppress each other’s ionisation (e.g., matrix effects). With ESI, proteins, carbohydrates, lipids, amino acids, polymers, etc., can be analysed.
- Atmospheric pressure chemical ionisation (APCI) is compatible with LC-MS, direct MS. The sample is introduced as liquid and sprayed into the source. The solvent evaporates and the compounds are converted to gas phase molecules by heat. The molecules are ionised by the reaction with ionic species created by the corona discharge. APCI operates at atmospheric pressure. With APCI, low to medium polarity compounds can be ionised. With APCI sample must be thermally stable and must be completely soluble in a volatile solvent. It is a soft and sensitive ionisation method, has quite high ionisation efficiency and compounds do not suppress each other’s (at least not so much as with ESI). It is suitable for samples with low analyte concentrations and different lipids, amino acids, resins, carbohydrates, etc., can be analysed.
- Matrix-assisted laser desorption/ionisation (MALDI) is compatible with direct LRMS and HRMS but not with LC-MS. MALDI is based on the desorption of a solid mixture of matrix substance and sample molecules and their ionisation by laser radiation whereby the matrix substance helps to ionise the sample molecules. Common matrix materials used in positive mode are dihydroxybenzoic acid (DHB), α-cyano-4-hydroxycinnamic acid (CHCA), sinapic acid (SA) and in negative mode pyridines, 3-aminoacridine (3-AA) and 9-aminoacridine (9-AA). MALDI enables the determination of compounds from very small samples (0.1 mg or even less) dissolved in a solvent (the sample does not need to be fully soluble) or just by implementing direct analysis, i.e. without dissolution and/or derivatisation of the sample. With MALDI, different complex, non-volatile, highly oxidized, non-soluble, and polymeric samples can be analysed. [3]
Mass analysers
The heart of a MS instrument is the mass analyser that separates the ions according to their m/z values. The separation is achieved by applying electric and/or magnetic fields to the formed ions. Mass analysers can be divided according to resolution. Low resolution (LR) MS instruments are, for example, quadrupole (Q) and ion trap (IT). High resolution (HR) MS devices are for example Fourier Transform Ion Cyclotron Resonance mass spectrometry (FT-ICR-MS) and Fourier Transform orbitrap (FT-OT). Time of Flight (ToF) can be classified as LR or HR depending on the specific configuration. [1,2]
Mass analysers (same type or different) can be also combined. These instruments are called tandem or hybrid or MS/MS instruments and they allow to do mass analysis in at least two stages. Nowadays, several tandem MS configurations are available, but the most common is the triple quadrupole mass spectrometer (TQMS or QQQ MS), where the first and third quadrupole mass analysers are used for selecting the ions and second quadrupole (nowadays also hexapole or octapole) is used for fragmenting the initial ions.
In Table 1 comparison of different mass analysers are presented.
Table 1. Comparison of mass analysers used for the analysis of cultural heritage materials. [3-5]
| Mass analyzer | Quadrupole (Q) | Ion trap (IT) | Time of Flight (ToF) | FT-ICR-MS | FT-OT-MS |
|---|---|---|---|---|---|
| Basic principle | – Ion separation obtained by using electric field. – Often used also as tandem instrument with three consecutive quadrupoles (QQQ, triple quad). – Can be combined with IT, ToF. | – Ions are separated by trapping them in an electric field and applying varying potential. – Can be combined with other mass analysers (Q, ToF, FT-ICR). | – Ions are accelerated and directed to a field-free zone where the ions are separated in time → ions with smaller m/z reach the detector faster. – Mostly reflectron (refl.) instruments are used → the filed-free zone is longer. – Can be combined with ToF, Q, IT. | – Ions are separated in magnetic field based on their cyclotron frequencies. – Can be combined with other mass analyzers (Q, IT). | Ions are separated in the electric field according to their cyclotron frequencies. |
| Resolution more info | Low (2000) | Low (4000) | ToF, linear: 5000 (low); ToF refl.: 30000 (high) | Very high (500000) | Very high (100000) |
| m/z accuracy more info | Low (100 ppm) | Low (100 ppm) | ToF, linear: 200 ppm; ToF refl.: 10 ppm | Very high (~1 ppm) | Very high (<5 ppm) |
| m/z range | Up to m/z 4000 | Up to m/z 6000 | ToF, linear: m/z >1000000; ToF refl.: m/z 10000 | Up to m/z 100000 | Up to m/z 50000 |
| Advantages | – Easy to use. – Very good detection limits. – Quite small and low-cost instrument. – Very good sensitivity for specifically selected ions. – Good compatibility with ESI, APCI, EI, CI. – Can be coupled with GC and LC. | – Quite small and low-cost instrument. – Relatively easy to use. – High sensitivity. – Good stability → mass spectra are reproducible. – Compatible with ESI, APCI, EI, CI, MALDI. – Can be coupled with GC and LC. | – Quick – the ions are scanned rapidly. – Medium-cost instrument with a relatively simple design. – High sensitivity. – Good compatibility with MALDI. – Can be used with EI, CI. – Can be coupled with GC and LC. | – Highest m/z accuracy and resolution. – Good sensitivity. – Very good for identifying complex materials. – Good compatible with ESI, APCI, MALDI, EI, CI sources. – Can be coupled with GC and LC. | – Offers very good m/z accuracy and resolution. – Very good for identifying complex materials. – Compatible with ESI, APCI, MALDI, EI, CI source. – Can be coupled with GC and LC. |
| Disadvantages | – Not suited for pulsed ion sources: MALDI. – Modest sensitivity for scanning all ions in a wider m/z range. | Ions are collected into the cell → overload of ions may occur which decreases the resolution. | More sophisticated equipment (HR ToF) is more expensive and requires skilled operator. | – The superconducting magnet makes the instrument big and expensive. – Complex device. – Requires very high vacuum (n x 10-10 bar). | Requires very high vacuum (n x 10-10 bar). |
Detectors
After sorting all the ions according to their m/z values, they are detected. This is usually done by converting the ions into electrical signals by an ion transducer. The more traditional detectors in mass spectrometry are the electron multiplier (the most widely used detector in different LR mass spectrometers, such as Q and QQQ, IT, ToF, etc.). For FT-ICR-MS and FT-OT-MS a different approach is used for detecting the ions. The ions are detected by detection plates that register the so-called image current that the ions produce while oscillating between these plates. [1,2]
Further reading
More information can be found on the University of Bristol, School of Chemistry Mass Spectrometry Facility web page:
- about ion sources: http://www.chm.bris.ac.uk/ms/ionisation.xhtml
- about mass analysers: http://www.chm.bris.ac.uk/ms/analysis.xhtml
- de Hoffmann, E.; Stroobant, V. Mass Spectrometry: Principles and Applications, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2007.
- Gross, J. H. Mass Spectrometry: A Textbook, 3rd ed.; Springer International Publishing: Switzerland, 2017.
- Colombini, M. P.; Modugno, F. Organic Mass Spectrometry in Art and Archaeology; John Wiley & Sons, Ltd.: Chichester, UK, 2009.
- Mazzeo, R. Analytical Chemistry for Cultural Heritage; Springer International Publishing: Switzerland, 2016.
- Teearu, A. Development of MALDI-FT-ICR-MS Methodology for the Analysis of Resinous Materials, PhD thesis; University of Tartu Press: Tartu, Estonia, 2017.
The slides used in the video can be downloaded from here:


