
MOOC: Instrumental analysis of cultural heritage objects
6.1. Chromatography
In this section, practical aspects and analysis with GC-MS/ FID and HPLC with different detectors are discussed, and examples are given.
Analysis with GC-MS and GC-FID methods
Gas chromatography (GC) is a widely used technique for the analysis of organic compounds of cultural heritage materials. The main detectors used for GC are mass spectrometric (MS) detector and Flame ionisation detector (FID) (more discussed in 5.1 Chromatography) that both enable qualitative and quantitative analysis. For the analysis of cultural heritage materials, GC-MS is the preferred technique because it enables a more reliable identification of the compounds present in the sample: a characteristic mass spectrum for every chromatographic peak is obtained. GC-FID lacks such a possibility: identifying is only possible when standards are used and identification is based on retention time (tR). Identification by retention time only is not very reliable as many compounds can have similar retention times.
The overall steps for GC-MS (and GC-FID) analysis:
- Sample preparation: depends on the material. Typically, it includes dissolving the sample or extracting the compounds with a suitable solvent (e.g. with hexane, toluene, etc.). During the sample preparation, derivatisation (CLICK HERE to read more about it) is often used to increase the volatility of the analytes and to enhance the chromatographic separation. With the multi-component mixtures, additional procedures may be needed, e.g. dilution, centrifugation, filtration of the solution, etc. Pyrolysis and SPME are special cases of sample preparation and their use is described below.
- In the case of quantitative analysis, an internal standard of known concentration is usually added to the sample solution. Internal standard is a compound that is chemically similar but not identical to the analyte(s). Also, calibration solutions containing the derivatives of the components of interest and internal standards must be made.
- Chromatographic analysis of the samples using the GC-MS (or GC-FID) instrument:
- Choosing a suitable method: Using a pre-existing method developed for a specific material type requires minimal (if any) parameter adjustments before analysis. Development of a new measurement method includes selecting a suitable column, adjusting the parameters, such as temperature program, injection volume, split ratio, etc. Method development is usually based on the analysis of standard materials.
- Injection of samples can be done manually with a micro syringe (one-by-one) or with an autosampler (if the instrument is equipped with it). The autosampler injects the sample(s) automatically according to the measurement series (sequence) set by the operator.
- Useful tips: Before the GC analysis, it may be beneficial to heat the column at a high temperature to clean the system. This should also be done after the analysis of a complex (real-life) samples. In the case of samples with unknown composition, it is advised to add blank injection(s) (e.g., injecting only the solvent) after the sample injection to make sure that the system is clean. Also, when starting work, it is useful to make a blank injection to verify that the system is clean.
- Interpretation of the results: For GC-FID the identification of the peaks on the chromatogram is based on the comparison of retention times of standard materials with the peaks obtained from the sample. In the GC-MS analysis beside chromatogram also mass spectra can be obtained – to each peak on the chromatogram corresponds also an averaged mass spectrum (see Fig. 1). For GC-MS, comprehensive commercial MS libraries are available that simplify the identification of the detected compounds.
The identification of the analysed material is usually based on the presence of specific biomarkers (e.g., abietic acid, dehydroabietic acid, and other resin acids for colophony resin), examining the molecular profile (e.g., the simultaneous presence of certain fatty acids, alcohols, and saturated hydrocarbons for beeswax) or by quantifying characteristic compounds (e.g., proteinaceous materials can be identified based on the quantities of specific amino acids).
In the following video, Dr Eliise Tammekivi demonstrates the usage of GC-MS and GC-FID in more detail.
In the video, as an example, the analysis of fresh linseed oil for determining its fatty acid composition was carried out. To make the analysis with GC possible, the oil components (triacylglycerides aka TAGs) were hydrolysed and then derivatised with TMTFTH (aka Meth-Prep II) reagent that produced a mixture of methylated fatty acids. These compounds are (differently from TAGs) sufficiently volatile for GC analysis. After the chromatographic run a chromatogram was obtained, where the compounds in the mixture were successfully separated and then identified (see Fig. 1). [1]
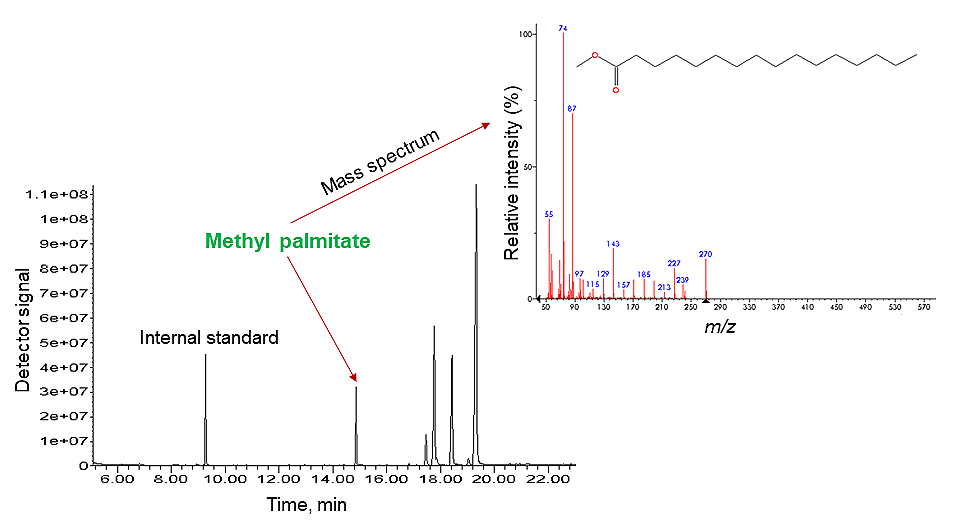
There are two sampling techniques that are commonly used in the field of cultural heritage with the GC-MS system: pyrolysis (Py) GC-MS and solid-phase microextraction (SPME) GC-MS.
Py-GC-MS is probably one of the most valuable tools for the analysis of various types of cultural heritage materials. In pyrolysis higher temperature (commonly over 600°C) can be used to desorb and pyrolyze the analysed compounds without extensive sample preparation. For cultural heritage materials, pyrolysis is often performed in the presence of a derivatisation reagent (thermally assisted derivatisation) to decrease the polarity of the compounds and thereby increase the volatility of the formed fragments, and to improve the separation of the analytes. Although pyrolysis essentially destroys the original material, the obtained pyrolysis products contain molecular markers that are used for the identification of the original material. [2]
SPME GC-MS can be used for a limited selection of materials. It is only used for the analysis of volatile or semi-volatile organic compounds (VOCs and SVOCs, respectively) coming from the object, like acetic acid, styrene, monoterpenes, alkanes, etc. Such compounds can be emitted by artefacts as a result of decomposition (ageing). The volatile compounds adsorbed in the SPME sampler can be injected into a GC using the same SPME sampler, without additional sample pre-treatment. [2,3]
Table 1 summarises some of the GC-MS/FID, Py-GC-MS, and SPME GC-MS possibilities for the analysis of different cultural heritage materials.
Table 1. Properties of GC-MS, Py-GC-MS, and SPME GC-MS for the analysis of cultural heritage materials [1–4].
| GC-MS/FID | Py-GC-MS | SPME GC-MS | |
|---|---|---|---|
| Analysed materials | Mostly natural (oils, waxes, resinous materials, dyes, proteins, polysaccharides) and some synthetic (e.g. alkyd) materials | Natural materials (oils, waxes, resinous materials, dyes, proteins, polysaccharides, lacquers), synthetic materials and polymers (alkyd, acrylics, vinyl resins, etc.) | VOCs, SVOCs, and volatile degradation products of natural resins, lacquers, some synthetic polymers (e.g., cellulose acetate, polyvinyl acetate), cellulose (e.g., paper, wood), oils, and waxes |
| Sample preparation | − Sample size: usually 0.1-20 mg. − Sample dissolved/extracted with organic solvent (methanol, ethanol, toluene, hexane, dichloromethane, etc.). NB! Water cannot be used. − Derivatisation is almost always required → choice of derivatisation reagent depends on sample type, pre-treatment, aim of analysis, etc. Main reagents: TMAH, TMTFTH aka Meth-Prep II, BSTFA, BSA. | − Sample size: normally 0.1-1 mg. − Sample preparation is not needed! − Sample placed into the pyrolyzer (heated chamber). If needed, derivatisation reagent (e.g., TMAH, HMDS) can be added. | − Sample size: larger than for GC-MS/FID and Py-GC-MS → only volatile compounds are analysed. − Sample preparation is not needed! − Derivatisation is not needed! − SPME fibre held near analysis object or sample for specific time (depends on analytes, size of object, etc.) to collect organic analytes. Fibre inserted to the GC injector where analytes are desorbed at high temperature and carried to the GC column. |
| Advantages | − Possible to analyse aged, oxidised, degraded materials. − Suitable for quantitative analysis (sample amount of at least 10 mg is often needed for reliable quantification). − Interpretation based on reference libraries. − Additional device is not needed for sample treatment (e.g,. pyrolyzer). − Gives more info than SPME-GC-MS. − The results are easier to interpret compared to Py-GC-MS. | − Capable of analysing essentially any organic material (e.g., natural and synthetic materials, polymers, etc.). − Sample pre-treatment is not needed. For some polymers derivtisation is not needed. − Possible to analyse aged, oxidised, degraded materials. − Possible to analyse very small samples. | − Easy interpretation – only volatiles compounds are analysed. − Non-destructive. − Sample pre-treatment, incl. derivatisation is not needed. − Possible to analyse aged, oxidised, degraded materials. − Possible to monitor the condition of objects (e.g., in museums). |
| Disadvantages | − Comprehensive sample preparation, e.g., hydrolysis, extraction, derivatisation, etc. − Not suitable for the analysis of most synthetic polymers. − Destructive method. Fragmenting technique → many peaks in the chromatogram make interpretation complicated | − Complex interpretation → very fragmenting technique. − Destructive method. − Side reactions, e.g., isomerisation of double bonds, etc. can occur. − Non-quantitative method due to the pyrolysis side reactions. | − Limited to volatile/semi-volatile analytes. − Requires more sample than GC-MS/FID or Py-GC-MS. − Possible to quantify volatiles near the object, but not in the object |
In conclusion, the choice of the GC column, detector, and method (parameters) depend on the analysable material and question in hand. If the components of the sample are sufficiently volatile (or can be made volatile by derivatisation), then GC-MS is the preferred method. When the sample contains polymers (incl. aged cultural heritage materials) that cannot be processed into suitable derivatives, then Py-GC-MS is the preferred approach. Both of these methods are destructive. SPME GC-MS is a non-destructive method that does not require sample preparation but is only suited for the analysis of VOCs and SVOCs of the object.
Analysis with HPLC using different detectors
Liquid chromatography (LC) is another separation technique that is widely used for the analysis of cultural heritage materials. With LC, qualitative and quantitative analysis can be carried out. Different materials require a different LC approach and occasionally quite extensive sample preparation. In this course, only the most common LC techniques have been discussed and the main focus being on reversed phase (RP) LC.
In LC, only solutions can be analysed. The sample must a) be dissolved in a suitable solvent or solvent mixture or b) it must be possible to extract the compound(s) from the sample into solution. Aged materials, such as paints, varnishes, etc. need usually a multi-step solvent extraction and/or derivatisation before they can be analysed with LC.
Overall steps during HPLC analysis:
- Sample preparation:
- Dissolving or extracting the sample with a suitable solvent/solvent mixture (choice of solvents depends on the material (see Table 2). Sample amount needed for LC analysis is roughly 1 mg of sample per 1 ml of solvent(s). With MS detector, the amount of sample can be lower than 1 mg (MS detectors are very sensitive). Optional additional procedures with the sample solution include dilution, derivatisation, adding internal standards (only needed for quantitative analysis), etc.
- Filtering or centrifuging of the sample solution. NB! This is a very important step in LC analysis and must not be skipped! The sample solution must not contain any solid particles to avoid contamination and damage to the LC system and column. For filtering, dense syringe filters are used. If the amount of sample solution is <50 μl, filtration is not the best solution because some of the solution will always remain in the filter. In that case, the solution is centrifuged and the clear supernatant is used for analysis.
- Preparation of additional standard solutions for analysis:
- Calibration standard solutions for quantitative analysis – solutions containing known different concentrations of compounds of interest.
- Reference standard solutions for qualitative analysis – solutions containing pure (certified) compounds. Reference standard solutions are measured under the same conditions as analysed sample and these results will help to identify and verify retention times and mass spectra of individual sample components.
- Measurement of samples:
- Choosing a suitable LC method: using a pre-existing method developed for the specific material type requires minimal, if any, parameter adjustments before analysis. Development of a new method involves selection of suitable solvent(s) and column; optimisation of LC parameters, such as flow rate, injection volume, eluent composition, etc. Method development is based on the analysis of reference materials. Most time-consuming and difficult step!
- Injection of samples: done manually or by an autosampler (more common nowadays) according to the set sequence.
- Useful tips for analysis (instrument maintenance): compared to GC, LC system needs more maintenance and has more parameters that need to be monitored/checked.
- For analysis, the LC system must be clean, have no leakages, blockages, or air trapped in the system (bubbles). To ensure this, the selected solvent(s) are run through the system and the pressure is monitored before injecting any samples. The instrument is ready for analysis when the system is conditioned with the solvents selected for the analysis, the system pressure and detector signal are stable.
- It is advised to add blank injections to the sequence (injection of only solvent or no sample at all) e.g., after each sample – this helps to keep the system clean and prevents carryover of compounds from one sample to the next.
- At the end of the analysis it is important to clean the system by rinsing with suitable solvent(s).
- To help protect and prolong the lifetime of the column, a guard column is used. It is a short column (usually 1 cm) with the same functionality as the main column. For UHPLC instruments, using an in-line filter before the column (or before the guard column) is advised, also. This will stop the solid particles that have entered the system and thus, prevents damage to the main column and instrument.
- Interpretation of results: for qualitative analysis retention times of peaks on the chromatogram belonging to the sample compounds are compared to retention times of the standard compounds. For quantitative analysis, a calibration graph is compiled (concentration of the compound vs peak area/height on the chromatogram) and from there the concentration for the compound can be calculated. In addition, different detectors can provide additional information about the sample compounds, e.g., with UV-VIS/PDA detector obtained UV-Vis absorbance spectra can give information whether a compound is coloured; mass spectrometric (MS) detector gives information about the mass, isotopic pattern, and chemical composition of the compounds, etc.
In the video below, Dr Pilleriin Peets gives a brief overview of how to carry out analysis with an HPLC system with different detectors on the basis of dye samples. In general, the analysis steps are similar to the analysis of other materials.
As explained in section 5.1 Chromatography different LC approaches are in use – reversed phase chromatography (RP), normal phase chromatography (NP), size exclusion chromatography (SEC), etc. RP is by far the most used LC technique and has been commonly applied for the analysis of a variety of cultural heritage materials. Also, SEC has been frequently used for cultural heritage materials, mainly for monitoring changes, degradation, and ageing of lipids, varnishes, polymers, etc. Up to now, NP chromatography has found limited use for the analysis of cultural heritage materials.
Table 2 presents an overview of the analysis of different materials with LC, focusing currently on the more common LC techniques.
Table 2. Analysis of different materials using more common LC techniques [2,5–7].
| LC separation technique | Sample preparation | Comments | |
|---|---|---|---|
| Textile dyes [8] | RP LC with C18 column → water mixed with methanol/acetonitrile as eluent system. | − Dye extracted with solvent(s): *direct dyes (some natural dyes e.g., safflower; synthetic azo dyes): MeOH, EDTA, DMSO; *mordant dyes (most natural dyes, e.g., madder): HCl diluted with H2O, MeOH; *vat dyes (e.g., indigo): DMF, DMSO, pyridine. − Multi-step extraction used, if needed. − Sample solvent(s) evaporated and extract re-dissolved in mobile phase. | − Different dyes need different LC methods → optimisation of LC conditions! − Suitable detectors: UV-Vis/PDA, MS(/MS); FLD is suitable only for some specific dyes (e.g., logwood). − Identification based on tR and spectral data of reference compounds. |
| Oils, fats, waxes (incl. archaeological residues, alkyd resins, etc.) [7] | RP LC with C18 column → isopropanol mixed with methanol, acetonitrile vs ethanol as mobile phase. NP LC is rarely applied and needs further testing. | − Applies for RP LC: sample dissolved/ extracted with suitable solvent(s): *oils, waxes, alkyd resins: hexane, tetrahydrofuran, n-heptane *fats: tetrahydrofuran, n-heptane, hexane, chloroform/methanol mixture. − Hydrolysis, derivatisation, and/or microwave extraction can be used (e.g., for aged samples). − Sample solvent(s) evaporated and extract re-dissolved in mobile phase. | − Fresh and modern materials can mostly be analysed. [9] − Analysis is problematic with aged and small samples → Py-GC-MS or direct MS are preferred. − Mostly MS(/MS) detector is used, UV-Vis/PDA is also suitable. − Identification and quantification are based on the analysis of TAGs in case of oils and fats. |
| Protein paint binders (egg yolk, egg white, also animal glues) [10] | RP LC with C18 column → acetonitrile mixed with water as mobile phase. SEC is used to monitor aging and changes in materials. [7] | − Extraction with ammonia, NaOH. − Enzymatic hydrolysis (with e.g., trypsin). − Drying and re-dissolving of sample in acidified water (or another suitable solvent). − Derivatisation with o-phthalaldehyde, etc. when using FLD and phenylisothiocyanate, etc. when using UV-Vis detector [11]. | − Suitable detectors: MS(/MS), UV-Vis/PDA, FLD. − Identification and quantification based on the analysis of amino acids → database needed. |
| Natural resins (colophony, dammar, shellac, etc.) [12] | RP LC with C18 column → acetonitrile/methanol mixed with water as mobile phase. NP LC is rarely applied and needs further testing. SEC is used to monitor degradation, changes in resinous materials. | Applies for RP LC: − Sample dissolved/extracted with methanol, isopropanol, ethanol, etc. − An ultrasonic bath (heated) is used if needed. | − Suitable detectors: UV-Vis/PDA, MS(/MS). − Identification based on standard/reference data. |
| Polysaccharides (wood, paper pulp, gums, etc.) [7] | SEC: gel with controlled pore size as stationary phase, usually tetrahydrofuran as mobile phase. RP/NP not suitable. | Tetrahydrofuran for dissolving and eluting of sample. | − Suitable detectors: UV-Vis/PDA, more specific detectors (e.g. refractive index detector (RID)). − Identification based on molecular weight distribution. − SEC suitable to monitor changes in materials (e.g. degradation of paper and wood, gums, etc.). |
| Synthetic polymers (PVA, acrylics, etc.) [6,7] | SEC: gel with controlled pore size as stationary phase, usually tetrahydrofuran as mobile phase. | Tetrahydrofuran for dissolving and eluting of sample. | − Suitable detectors: UV-Vis/PDA, also more specific detectors, like refractive index detector (RID) are used. − Possible to study molecular weight distribution → describes the composition, decomposition state, age, etc. − Identification is based on standard/reference data. |
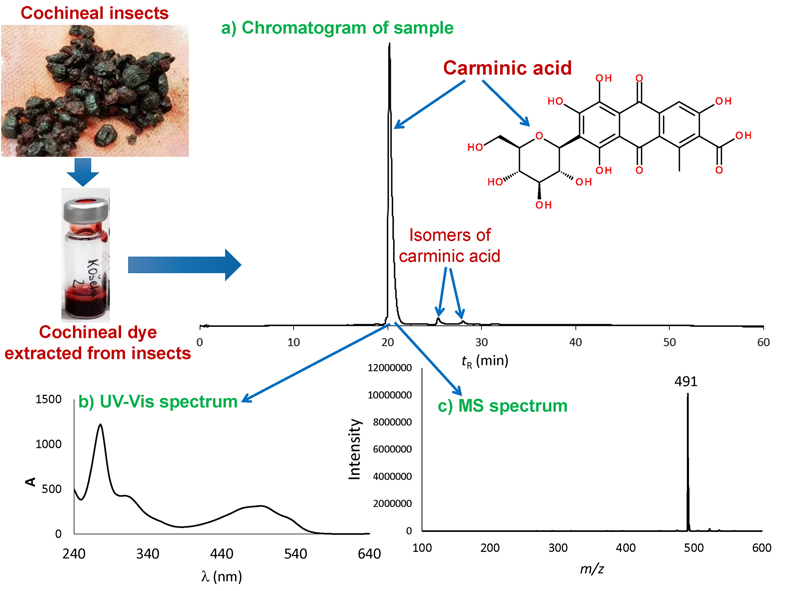
As mentioned above, LC systems with different detectors give different type of information. In Fig. 2, an example of an analysis result of cochineal extract obtained with LC instrument coupled with UV-VIS/PDA and MS detectors is presented. This means that in addition to the chromatogram (Fig. 2a) also in case of UV-VIS/PDA, the UV-Vis spectrum (Fig. 2b) and with MS detector a mass spectrum (Fig. 2c) corresponding to a specific chromatographic peak can be obtained.
- Tammekivi, E.; Vahur, S.; Kekišev, O.; van der Werf, I. D.; Toom, L.; Herodes, K.; Leito, I. Comparison of Derivatization Methods for the Quantitative Gas Chromatographic Analysis of Oils. Anal. Methods. 2019, 11 (28), 3514–3522. https://doi.org/10.1039/C9AY00954J.
- Colombini, M. P.; Modugno, F. Organic Mass Spectrometry in Art and Archaeology; John Wiley & Sons, Ltd.: Chichester, UK, 2009.
- Bonaduce, I.; Ribechini, E.; Modugno, F.; Colombini, M. P. Analytical Approaches Based on Gas Chromatography Mass Spectrometry (GC/MS) to Study Organic Materials in Artworks and Archaeological Objects. Top. Curr. Chem. 2016, 374 (1), 6, 1-37. https://doi.org/10.1007/s41061-015-0007-x.
- Hübschmann, H.-J. Handbook of GC/MS. Fundamentals and Applications, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009.
- Stuart, B. H. Analytical Techniques in Materials Conservation; John Wiley & Sons, Ltd.: Chichester, UK, 2007.
- Mazzeo, R. Analytical Chemistry for Cultural Heritage; Springer International Publishing: Switzerland, 2016.
- Degano, I.; La Nasa, J. Trends in High Performance Liquid Chromatography for Cultural Heritage. Top. Curr. Chem. 2016, 374 (2), 20, 1-20. https://doi.org/10.1007/s41061-016-0020-8.
- Peets, P.; Vahur, S.; Kruve, A.; Haljasorg, T.; Herodes, K.; Pagano, T.; Leito, I. Instrumental Techniques in the Analysis of Natural Red Textile Dyes. J. Cult. Herit. 2020, 42, 19–27. https://doi.org/10.1016/j.culher.2019.09.002.
- La Nasa, J.; Zanaboni, M.; Uldanck, D.; Degano, I.; Modugno, F.; Kutzke, H.; Tveit, E. S.; Topalova-Casadiego, B.; Colombini, M. P. Novel Application of Liquid Chromatography/Mass Spectrometry for the Characterization of Drying Oils in Art: Elucidation on the Composition of Original Paint Materials Used by Edvard Munch (1863–1944). Anal. Chim. Acta. 2015, 896, 177–189. https://doi.org/10.1016/j.aca.2015.09.023.
- Vinciguerra, R.; De Chiaro, A.; Pucci, P.; Marino, G.; Birolo, L. Proteomic Strategies for Cultural Heritage: From Bones to Paintings. Microchem. J. 2016, 126, 341–348. https://doi.org/10.1016/j.microc.2015.12.024.
- Prikryl, P.; Havlíčková, L.; Pacáková, V.; Hradilová, J.; Štulík, K.; Hofta, P. An Evaluation of GC-MS and HPLC-FD Methods for Analysis of Protein Binders in Paintings. J. Sep. Sci. 2006, 29 (17), 2653–2663. https://doi.org/10.1002/jssc.200600171.
- van der Doelen, G. A.; van den Berg, K. J.; Boon, J. J.; Shibayama, N.; de la Rie, E. R.; Genuit, W. J. L. Analysis of Fresh Triterpenoid Resins and Aged Triterpenoid Varnishes by High-Performance Liquid Chromatography–Atmospheric Pressure Chemical Ionisation (Tandem) Mass Spectrometry. J. Chromatogr. A. 1998, 809 (1–2), 21–37. https://doi.org/10.1016/S0021-9673(98)00186-1.


