
MOOC: Instrumental analysis of cultural heritage objects
Paper
Since ancient times, before the discovery of paper, people have been writing on different materials: stone, tree bark, papyrus plants (Cyperus papyrus), parchment (vellum), etc. Parchment was produced from the dermis of animal skin. Papyrus is composed mainly of cellulose (around 53–62%) and lignin (22–33%), and the main component of parchment is collagen [1,2].
However, probably the most important writing material is paper. It was invented in China 2-1 century BCE and papermaking was introduced to Europe (Spain) by Arabs around the 12th century [3,4]. By definition, paper is composed of matted or felted sheets usually made of cellulose fibres, formed on a wire screen from water suspension, followed by pressing and drying [5]. Paper is a multi-component material and its behaviour (chemical and mechanical properties, stability, resistance to degradation, etc.) depends upon the nature and characteristics of the paper components and upon their interactions [6,7]. Fig. 1 generally summarizes the main components of the paper [6-8].
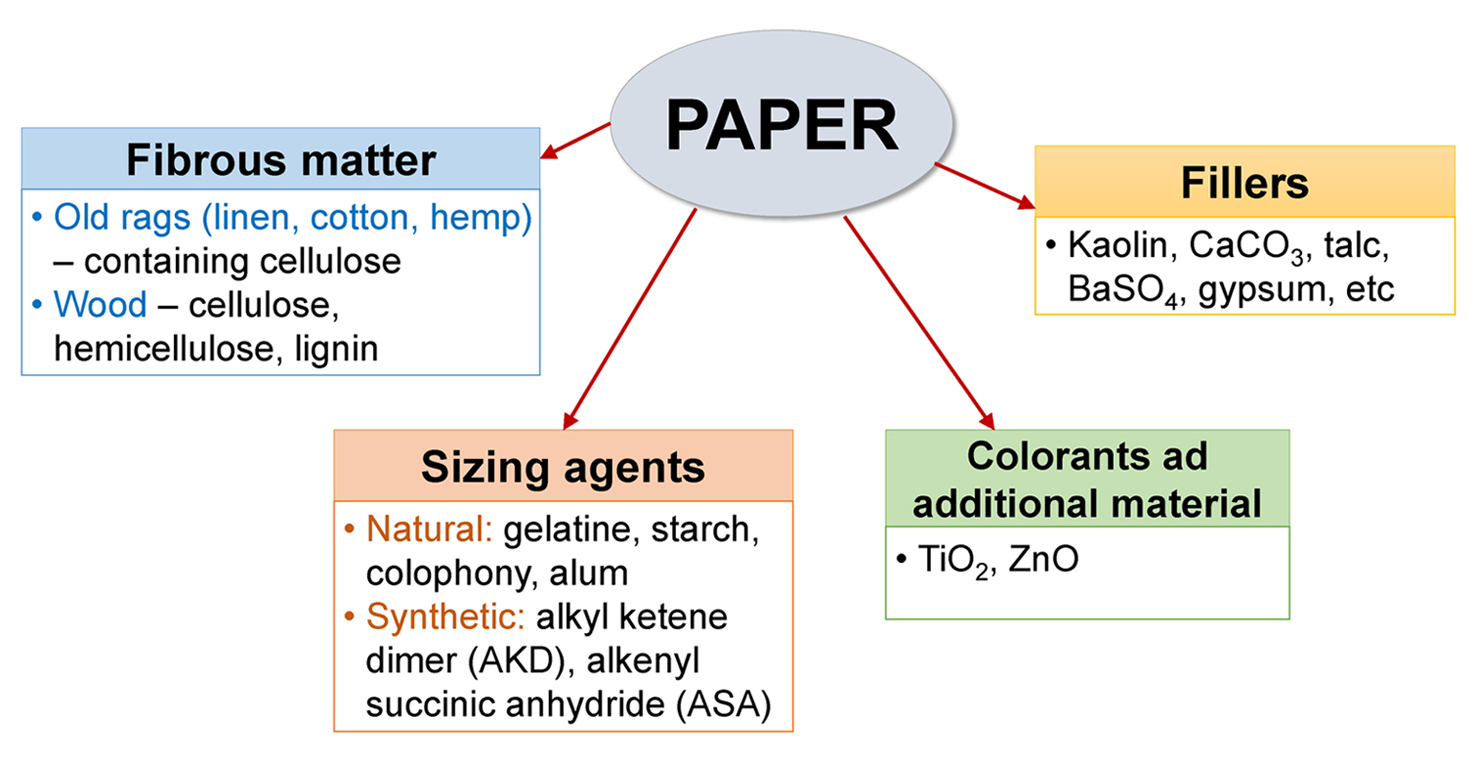
Early European papers (before the mid-19th century) were made from old rags (linen, cotton, hemp) that consist mostly of pure cellulose. In order to make the paper surface less absorptive gelatine or starch flour was added as a sizing agent. Sometimes no sizing was used at all (often with hand-made paper) [3,7]. Lime (calcium hydroxide) was usually added to the fibrous slurry before the beating process. It reacts with CO2 from the air and forms calcium carbonate, which afterwards provides the alkaline reserve for the paper. The alkaline reserve is an important property of paper, defining its long-term ability to withstand acidic degradation (see below). Due to that, old papers can show alkaline reaction even today [6].
In the middle of the 19th century, signficant changes took place in the technology of papermaking: wood-derived fibres and alum (Al2(SO4)3·nH2O))-rosin sizing were taken into use [7,9]. Due to the hemicellulose and lignin in the wood cellulose pulp, the quality of paper decreased considerably. Due to the influence of oxygen from the air, especially in the presence of even a small amount of transition metal ions, lignin oxidises easily to compounds containing chromophores and carboxyl groups. This process leads to yellowing of paper and degradation of its mechanical strength because the formed acid contributes to the hydrolysis of cellulose. Also, lignin absorbs UV radiation and makes the paper sensitive to sunlight [6,10–12] Fig. 2a and Fig. 2b presents paper made from old rags and paper from wood-pulp.

Mould damage of rag paper. Possible causes: high humidity and temperature, microorganisms.
(Photo from the National Archives of Estonia)

Browning, yellowing of lignin-containing groundwood paper. Possible causes: light and also contact with acidic material. (Photo from the National Archives of Estonia)
In the industry, different pulping processes (sulfite process, kraft process, soda pulping, etc.) have been taken into use for removing lignin and separating it from cellulose. Also, in the wood pulp mixture started to add to the pulp or paper surface different fillers and colorants (calcium carbonates, kaolin, gypsum, barium sulphate, titanium dioxide, etc.). The fillers and sizing agents have been used to neutralise the paper from the acidity and/or to improve printability, writability, brightness, opacity and smoothness and also to reduce paper production cost [13,14]. Due to the low cost, availability and favourable properties, calcium carbonate (CaCO3) and kaolin (Al2Si2O5(OH)4) have been the most commonly used fillers in the paper. For making paper different forms of calcium carbonate have been used: CaCO3 that is available from chalk deposits, precipitated calcium carbonate (PCC), etc. [15]. The use of kaolin (mineral called kaolinite) started already in the 18th century, and the use of CaCO3 became dominant in the 20th century when it was established as the acid-neutralising agent in archival papers. The amount of kaolin and/or calcium carbonate can reach almost 40% by mass of the content of the sheet in the case of filled papers and can exceed 50% for coated papers [6,16].
Nowadays, most book paper is made of different combinations of wood pulp, de-inked wastepaper etc. In addition to pulp, it contains various amounts of sizing, fillers, and dyes [17]. So the quality and composition of paper of different books can vary widely.
Nowadays, besides uncoated papers, there are different types of coated papers: gloss and matt papers, plastic-coated and photographic papers, etc. Coated paper has a thin layer of material or mixture of materials or a polymer applied to one or both sides to give specific qualities to the paper: durability, surface gloss, smoothness, or reduce ink absorbency. For coating printing paper, minerals like calcium carbonate (chalk), kaolinite (china clay), titanium dioxide, bentonite, talc etc. and binders (sizing agents) like starch, casein, acryl-butadiene, styrene-butadiene latexes, polyvinyl alcohol, etc. with different dispersants, plasticizers, etc. have been used [18]. For making photographic paper polyethylene or polyolefin extrusion coating, silicone, and wax coating have been used [19]. Plastic-coated paper or paperboard is mostly used for making food and drink packaging. The polymer (plastic) is used to improve functions such as water and abrasion resistance, the ability to be heat sealed, etc. Mostly polyethylene (LDPE) and sometimes polyethylene terephthalate (PET) have been used. For example, the paperboard package for wine contains about 74% paper, 22% plastic and 4% aluminium [20].
Requirements for the permanence of papers
The resistance of paper to ageing is important both for the documents stored in the memory institutions, as well as for different enclosures made of paper. The international EN/ISO 9706 standard “Information and documentation – Paper for documents – Requirements for permanence” defines the specifications for permanent, durable paper, which should be made of quality bleached chemical pulp with the absence of mechanical pulp (Kappa number should attain values lower than 5, which means that the paper may contain only a small amount of easily oxidized material (e.g. lignin)) and neutrally sized. In addition, it should contain as minimum 2% by mass of calcium carbonate filler, acting as an alkaline reserve (alkali reserve at least corresponding to 0.4 mol of acid per kg of paper). The pH should be in the range of 7.0 to 10.0 and tearing resistance of at least 350 mN. Optical bleaching agents are not allowed.
There are also standards for archival paper (ISO 11108) and for boxes, covers and other enclosures, made from cellulosic materials for storage of paper and parchment documents (ISO 16245).
In conclusion, the significance of any paper item should not be underestimated: everything, from works of art to the ephemera clutter of everyday life, adds to the broader picture of the past lives of our ancestors.
Further reading:
- European Papermaking Techniques 1300–1800, Timothy Barrett: http://paper.lib.uiowa.edu/european.php
- Encyclopedia Britannica: https://www.britannica.com/technology/paper
- Encyclopedia Britannica: https://www.britannica.com/technology/papermaking
- Stuart, B. Analytical Techniques in Materials Conservation; John Wiley & Sons: Chichester, England ; Hoboken, NJ, 2007.
- Taha, A. S.; Salem, M. Z. M.; Abo Elgat, W. A. A.; Ali, H. M.; Hatamleh, A. A.; Abdel-Salam, E. M. Assessment of the Impact of Different Treatments on the Technological and Antifungal Properties of Papyrus (Cyperus Papyrus L.) Sheets. Materials 2019, 12 (4), 620. https://doi.org/10.3390/ma12040620.
- Hunter, D. Papermaking: The History and Technique of an Ancient Craft; Dover Publications: New York, 1978.
- Bloom, J. M. Paper Before Print: The History and Impact of Paper in the Islamic World, First Printing edition.; Yale University Press: New Haven, 2001.
- paper | Definition, Papermaking, & Facts https://www.britannica.com/technology/paper (accessed Oct 31, 2020).
- Vahur, S.; Eero, L.; Lehtaru, J.; Virro, K.; Leito, I. Quantitative Non-Destructive Analysis of Paper Fillers Using ATR-FT-IR Spectroscopy with PLS Method. Anal. Bioanal. Chem. 2019, 411 (20), 5127–5138. https://doi.org/10.1007/s00216-019-01888-x.
- Area, M. C.; Cheradame, H. Paper Aging and Degradation: Recent Findings and Research Methods. BioResources 2011, 6 (4), 5307–5337.
- Collings, T.; Milner, D. A New Chronology of Papermaking Technology. Pap. Conserv. 1990, 14 (1), 58–62. https://doi.org/10.1080/03094227.1990.9638387.
- Cséfalvayová, L.; Pelikan, M.; Kralj Cigić, I.; Kolar, J.; Strlič, M. Use of Genetic Algorithms with Multivariate Regression for Determination of Gelatine in Historic Papers Based on FT-IR and NIR Spectral Data. Talanta 2010, 82 (5), 1784–1790. https://doi.org/10.1016/j.talanta.2010.07.062.
- Carter, H. A. The Chemistry of Paper Preservation: Part 1. The Aging of Paper and Conservation Techniques. J. Chem. Educ. 1996, 73 (5), 417. https://doi.org/10.1021/ed073p417.
- Carter, H. A. The Chemistry of Paper Preservation: Part 2. The Yellowing of Paper and Conservation Bleaching. J. Chem. Educ. 1996, 73 (11), 1068. https://doi.org/10.1021/ed073p1068.
- Šelih, V. S.; Strlič, M.; Kolar, J.; Pihlar, B. The Role of Transition Metals in Oxidative Degradation of Cellulose. Polym. Degrad. Stab. 2007, 92 (8), 1476–1481. https://doi.org/10.1016/j.polymdegradstab.2007.05.006.
- Industrial Minerals & Rocks: Commodities, Markets, and Uses, 7th Edition; Kogel, J. E., Trivedi, N. C., Barker, J. M., Krukowski, S. T., Eds.; SME, 2006.
- Shen, J.; Song, Z.; Qian, X.; Ni, Y. A Review on Use of Fillers in Cellulosic Paper for Functional Applications. Ind. Eng. Chem. Res. 2011, 50 (2), 661–666. https://doi.org/10.1021/ie1021078.
- Carter, H. A. The Chemistry of Paper Preservation: Part 4. Alkaline Paper. J. Chem. Educ. 1997, 74 (5), 508. https://doi.org/10.1021/ed074p508.
- Dabrowski, J. Fibre Loading in Papermaking. Pap. Hist. 2009, 13 (1), 6–11.
- Papermaking | Process, History, & Facts https://www.britannica.com/technology/papermaking (accessed Oct 31, 2020).
- Konsa, K. Arhivaalide Ja Trükiste Säilitamine; Lepp, A., Ed.; Ajalookirjanduse Sihtasutus “Kleio”: Tartu, 2008.
- Pénichon, S. Twentieth-Century Color Photographs: Identification and Care; Getty Conservation Institute: Los Angeles, California, 2013.
- Blunt, L. Recycling Mystery: Milk and Juice Cartons. Earth 911, 2018.


