
MOOC: Instrumental analysis of cultural heritage objects
6.2. Mass spectrometry
This section summarises practical analysis aspects of mass spectrometry, and guidelines on choosing a suitable ion source and mass analyser combination for investigating different materials are given.
Practical aspects of direct MS
Mass spectrometry is a powerful technique that provides valuable molecular information about the components of complex materials. It is often combined with gas chromatography (GC) or liquid chromatography (LC). It is also applicable as a stand-alone (direct) technique allowing to combine different ionisation methods (electrospray ionisation (ESI), atmospheric pressure chemical ionisation (APCI), matrix-assisted laser desorption ionisation (MALDI), etc.) and mass analysers (quadrupole (Q), ion trap (IT), Fourier transform ion cyclotron resonance (FT-ICR), time of flight (ToF), etc.).
Direct MS can be very useful in the analysis of cultural heritage samples. The main advantages of direct MS compared to LC-MS and GC -MS analysis are:
- Possibility to use smaller sample amounts: about a few µg or even less for direct MS;
- Simpler sample preparation: possible to use a wider selection of solvents (dichloromethane, acetone, methanol, etc.), no special sample pre-treatment (derivatisation, etc.) needed and no limitations that could come from the column or chromatographic system;
Possible to perform analysis directly (i.e., without dissolution, extraction) from the solid samples (like aged adhesive residues, dyes from textile fibres, etc.). This kind of analysis can be made with MALDI-MS, direct temperature MS techniques, such as Direct Inlet-Mass spectrometry (DI-MS), Direct Exposure-Mass Spectrometry (DE-MS), and Direct Temperature Resolved-Mass Spectrometry (DTMS).[1]
The list of different MS instruments is long. Essentially, all the ion sources can be combined with the available mass analysers, but some combinations have proven more efficient and easier to manage. E.g., the MALDI source is very suitable for ToF, while ESI and APCI work well with QQQ. Another prominent class of direct MS techniques are the direct temperature MS techniques, like DTMS, DI-MS, and DE-MS, which use high temperature to desorb/pyrolyze the analysed compounds. These techniques use the EI/CI source (also widely used for GC-MS).
From the practical aspect, the choice of the ion source is very important, and it is mainly determined by the type and properties of the sample. Based on the sample, the following aspects should be considered: (1) in which solvents (if at all) and how well the sample dissolves, (2) whether it ionizes better in the positive or negative mode, (3) whether the material consists of small or larger molecules, (4) sample amount available, etc. For example, for ESI and APCI sources, the sample must be completely dissolved, but with MALDI also hazy solutions and even solid samples can be directly analysed.
The overall steps of direct MS analysis are the following (see more information in Table 1):
- Sample preparation (depends greatly on the instrument, ion source): for ESI/APCI-MS and also for MS with EI/CI the sample must be dissolved/extracted. Filtration, sometimes dilution, rarely derivatisation may be needed. For MALDI-MS and also for high-temperature direct MS techniques, the sample can be solid or partially dissolved.
- For the analysis with HRMS instruments, calibration standards should be used to calibrate the m/z axes of the measured MS spectra to obtain the highest m/z accuracy possible (e.g., m/z errors less than 2 ppm) [2-5]. For this, a solution of calibration standards, also named internal standards (at least 3 compounds), is added to the sample during the preparation (usually calibration solution is mixed with sample solution).
- Measurement of the sample with MS:
- Introduction of the sample into the ion source. Usually, a sample solution is introduced into the ion source with a micro syringe (with ESI-, APCI-MS), using a special target plate (with MALDI-MS) or direct inlet probe (with DTMS, DI-MS and DE-MS).
- For external calibration of the HRMS instrument (to verify the overall conditions of the HRMS instrument), the calibration standard solution should be analysed similarly as the sample.
- Optimisation of the parameters of ion source and mass analyser. For that, special software of the instrument is used. Usually, it is done once during method development and then adjusted if needed. If all the parameters are fixed, then the mass spectrum of the sample is recorded.
- Interpretation of results. Interpretation of mass spectrum is based on m/z values and the corresponding ion formulas. See more details below.
Please see Table 1 and the videos below for more detailed information. The information in Table 1 is a very broad generalisation. Exceptions can be found in almost all statements presented in the table.
Table 1. Comparison of some mass spectrometric techniques based on the ion source and their suitability for the analysis of cultural heritage materials. Under the ion source (in bold), in brackets are the more common mass analysers that are combined with the source. [1,6]
| EI and CI (+ Q, IT) | MALDI (+ToF, FT-ICR, also Q, QQQ, IT and combinations) | ESI and APCI (+QQQ, IT, FT-OT, FT-ICR, also ToF and combinations) | |
|---|---|---|---|
| Sample preparation | − Gas-phase ion source → analytes are introduced in gas phase. − Mainly used (a) in hyphenation with gas chromatography, as detector; (b) with direct temperature MS techniques: dissolved/extracted or solid sample is inserted into the ion source (through a specific inlet) and gradually heated → compounds are desorbed/pyrolyzed. − Suitable solvents: methanol, ethanol, toluene, DCM, etc. | − The sample is co-crystallized with a matrix material: sample (solution) is mixed with matrix solution and placed on MALDI target plate, solvent(s) is (are) evaporated, and dried crystals are analysed. − The sample does not need to be fully soluble (the solutions can be hazy). − Matrix substances: *positive ion mode: DHB, CHCA, SA, etc. *negative ion mode: 9-AA, 3-AA, etc. − Suitable solvents: water, methanol, toluene, DCM, DMSO, etc. | − Liquid phase ion source → analytes are introduced in liquid phase, most often in combination with liquid chromatography in MS detector. − Sample is dissolved/extracted with solvent(s). Sample solution must be filtered (or centrifuged)! − Suitable solvents: ESI: most commonly methanol, acetonitrile, water, and their mixtures; less often toluene, ethyl acetate, dichloromethane, tetrahydrofuran, etc. APCI: methanol, hexane, acetonitrile, dichloromethane, etc. |
| Sample size | Less than 1 µg, usually only few ng of a compound in a mixture. | Less than 1 µg (per mL of sample solution). | ESI and APCI: approx. 1 mg per mL of sample solution. Nano-ESI: few dozen µg per mL of sample solution. |
| Material types that can be analysed | Compounds with sufficient vapor pressure (boiling points below ca 600 °C) directly; non-volatile compounds, such as resinous materials (resins, varnishes, tars), oils/fats, waxes, saccharides, proteins/peptides, dyes, polymers can be analysed using derivatization and/or pyrolysis. | Resinous materials (resins, varnishes, tars), oils/fats, waxes, saccharides, proteins/peptides, dyes, polymers, inorganic compounds. | ESI: Resinous materials (resins, varnishes, tars), oils/fats, waxes, saccharides, proteins/peptides, dyes, polymers. APCI: Resinous materials (resins, varnishes, tars), oils/fats, waxes, saccharides, dyes. |
| Information in mass spectra (more info) | EI: − Highly fragmenting → fragment ions are detected, sometimes also the molecular ion → characteristic fragmentation pattern for compounds. − Only positive ions are generated. CI: − Less fragmenting → molecular ions and fragment ions → simple mass spectrum − Adduct ions with the reagent gas may form. − Positive and negative ions can be generated. | − Positive and negative ions can be generated → mainly adduct ions with H+, Na+, K+, Cl–, etc. → characteristic to specific materials. − Mainly singly charged ions. − Low fragmentation. − Macromolecules can be ionised. | − Positive and negative ions. − Low fragmentation. ESI: − Singly and multiply charged ions → multiply charged ions: analysis of compounds with very high mass (approx. 100000 Da), singly charged ions: information about small molecules. − Generates mainly adduct ions with H+, Na+, K+, Cl–, etc. − Preferably more polar compounds are ionised. APCI: − Mainly singly charged ions. − Mainly protonated or deprotonated ions. − Preferably less polar to nonpolar small (up to 2000 Da) compounds are ionised. |
| Advantages | − Very small sample can be analysed → very sensitive. − Very good ionisation efficiency. − Fragmentation leads to characteristic spectra (“fingerprints”) and gives information about the structure of the compounds. − Extensive and well accessible spectral libraries exist that are help with identification. − Qualitative and quantitative analysis. | − Can be used for partially dissolved samples (hazy solution) or directly on the solid samples without extraction/decomposing − Easy sample preparation. − Aged, oxidised, degraded samples can be analysed, incl. materials that are (partly) polymeric and non-volatile. − No extensive fragmentation → typically ions corresponding to original compounds and their derivatives are detected − Good ionization efficiency. | − Soft ionisation. Qualitative and quantitative analysis. ESI: − Suitable for the analysis of most organic compounds. − Suitable for analytes with low concentration (in case of nano-ESI). APCI: − Less matrix effects compared to ESI. |
| Disadvantages | − Molecules can be extensively fragmented (e.g., aliphatic compounds) and spectra may be insufficiently characteristic for identification. In particular, molecular ions are not always observed. − Instrument needs constant monitoring and maintenance. − Skilled operator is needed. − CI → limited amount of structural information. | − Selection of matrix substance and suitable solvents is crucial → the matrix and solvent(s) must be compatible with sample. − Poorly reproducible spectra. − Not suitable for quantitative analysis. − Interpretation relies on comparison to standard materials and literature. − Instrument needs constant monitoring and maintenance. − Skilled operator is needed. | − Liquid environment for the ionisation needed → Sample has to be dissolved (only the dissolved/extracted part of the sample can be analysed). − Higher sample amount needed. − Instrument needs constant monitoring and maintenance. − Skilled operator is needed. ESI: − Matrix effects − Spray stability depends on the solvent. APCI: − Only suitable for small molecules (under 2000 Da). − System needs constant cleaning → contamination of the corona needle and MS inlet. |
As demonstrated in Table 1, the information obtained with different MS instruments can vary because the ionisation method is different. As an example, in Fig. 1, mass spectra of shellac resin, colophony resin (both in positive mode) and extract of dyer’s madder plant (negative mode) obtained with FT-ICR-MS coupled to MALDI, APCI and ESI sources, respectively are presented. The selection of ion mode – positive or negative depends on the analysed material (e.g., natural resins preferably give positive ions while dyes give negative ions). In positive mode, ESI and APCI give mostly protonated ions, sometimes also adducts with Na+. With MALDI, preferably adducts with Na+, sometimes also K+, Al+, etc., are observed (such adducts are common for compounds with a higher amount of oxygen in them). In negative ion mode, mainly deprotonated ions are observed but also the addition of Cl– or other anions may be possible (see Fig.1c). With ESI, more polar compounds can be detected (reins, dyes, oils/fats, etc.), MALDI and APCI are also suitable for less polar compounds (waxes, hydrocarbons).
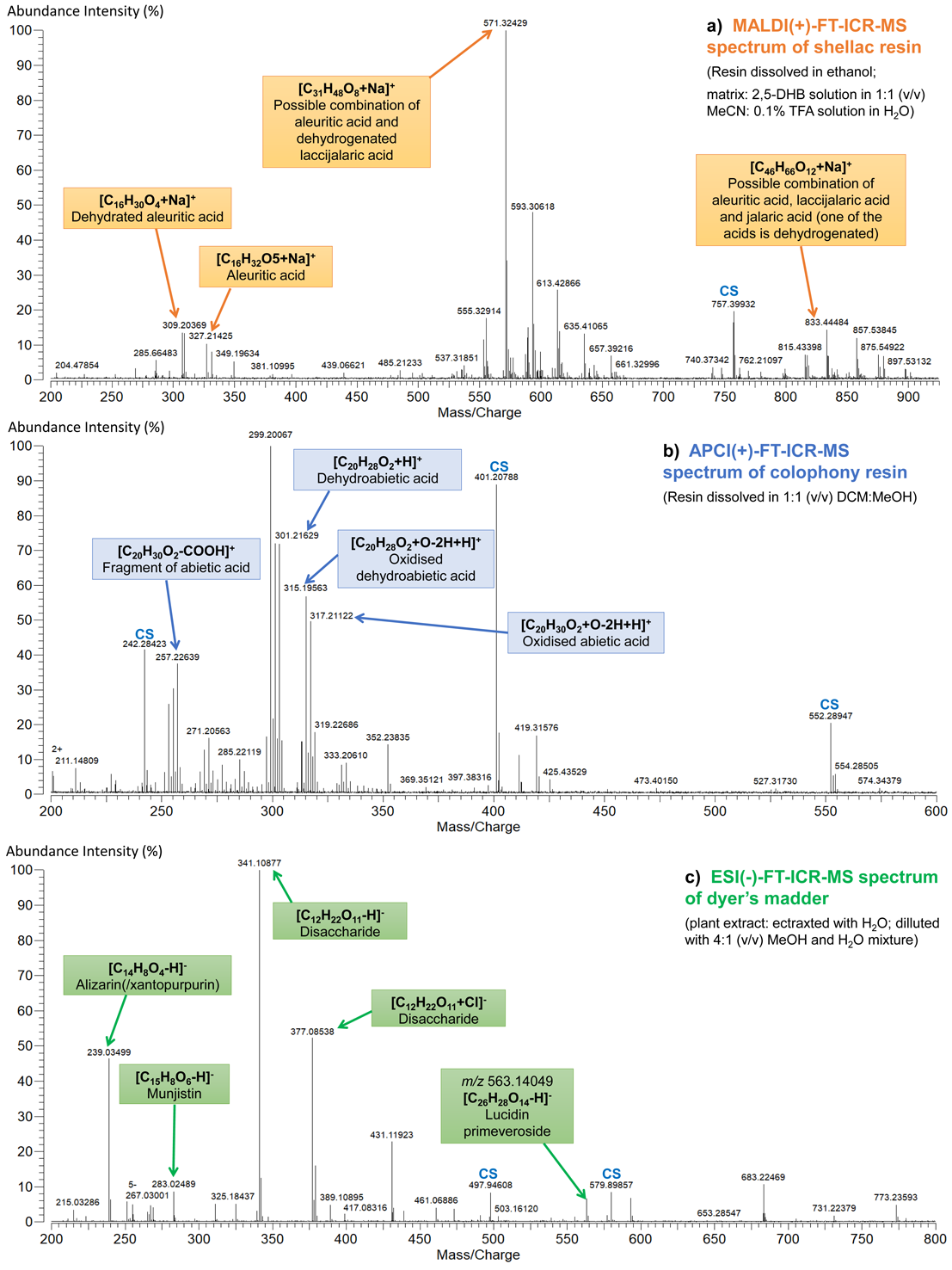
Interpretation of the obtained mass spectra is based on the identification of detected ions/fragments and also on the isotope pattern, which helps to determine the charge of the ion (by the spacing between the satellite peaks), as well as the presence of some elements with characteristic isotope patterns (such as Cl, Br, S). If a HRMS instrument is used, like FT-ICR-MS, Orbitrap, or high-end ToF, then the obtained m/z values are very accurate (four to six decimals) and often a single mass analyser is enough for assigning correct ion formulas and, hence, identifying the corresponding compounds, as demonstrated on Fig.1 (reference mass spectra are still very helpful). With LRMS instruments only nominal m/z values are obtained. This makes m/z-based identifications difficult or impossible because the number of potential ion formulas corresponding to a nominal value can be very high. In the case of LRMS, tandem instruments (see section 5.2) are helpful. This technique allows to select specific m/z values, fragment them, and analyse the obtained fragments. In this case, identification is mostly based on fragmentation patterns – different compounds (even with the same nominal m/z value) can fragment differently.
Analysis with MALDI-, ESI- and APCI-FT-ICR-MS
In the following video, Dr Anu Teearu-Ojakäär introduces the principles of MALDI-FT-ICR-MS, sample preparation and how to perform analysis with this instrument.
In the following video, Dr Anu Teearu-Ojakäär introduces the FT-ICR-MS with APCI and ESI ionisation sources, sample preparation and how to perform analysis with ESI-FT-ICR-MS.
- Colombini, M. P.; Modugno, F. Organic Mass Spectrometry in Art and Archaeology; John Wiley & Sons, Ltd.: Chichester, UK, 2009.
- Teearu, A. Development of MALDI-FT-ICR-MS Methodology for the Analysis of Resinous Materials, PhD thesis. University of Tartu Press: Tartu, Estonia, 2017.
- Teearu, A.; Vahur, S.; Rodima, T.; Herodes, K.; Bonrath, W.; Netscher, T.; Tshepelevitsh, S.; Trummal, A.; Lõkov, M.; Leito, I. Method Development for the Analysis of Resinous Materials with MALDI-FT-ICR-MS: Novel Internal Standards and a New Matrix Material for Negative Ion Mode. J. Mass. Spectrom. 2017, 52 (9), 603–617. https://doi.org/10.1002/jms.3943.
- Teearu, A.; Vahur, S.; Haljasorg, U.; Leito, I.; Haljasorg, T.; Toom, L. 2,5-Dihydroxybenzoic Acid Solution in MALDI-MS: Ageing and Use for Mass Calibration. J. Mass. Spectrom. 2014, 49 (10), 970–979. https://doi.org/10.1002/jms.3395.
- McIver, R. T.; McIver, J. R. Fourier Transform Mass Spectrometry; IonSpec Corporation: Lake Forest, CA, USA, 2006.
- Stuart, B. H. Analytical Techniques in Materials Conservation; John Wiley & Sons, Ltd.: Chichester, UK, 2007.


