
MOOC: Instrumental analysis of cultural heritage objects
7.2. Case study 2
Analysis of figurine Gertrud from the Retable of the High Altar of Tallinn’s St. Nicholas’ Church
Text Author: Assoc. Prof. Dr. Signe Vahur (signe.vahur@ut.ee)
1. Analysed object and aim of the research
During 2013-2016 the cultural heritage research group at the chair of Analytical Chemistry participated in a comprehensive research project called “Rode Altarpiece in Close-up” related to the history, technical investigation and conservation of the Retable of the High Altar of St. Nicholas’ Church in Tallinn. This project was initiated by the Niguliste Museum, one of the branches of the Art Museum of Estonia. Besides us, other institutions like the Estonian Academy of Arts, Conservation Centre Kanut, Estonian Environmental Research Centre, University of Southampton and Estonian Tax and Customs Board were involved. From the investigation results, a book called „Rode Altarpiece in Close-up” was published [1], and also webpage was created (http://rode.ekm.ee/index-est.html).
Retable is one of the most magnificent cultural heritage masterpieces in Estonia and possibly the best preserved late medieval Northern German altarpiece in Europe. The retable was probably made in the workshop of the Lübeck master Hermen Rode in 1481. More than forty saints and biblical figures are depicted in the retable, and its dimensions place it among the largest retables from the 15th-century Hanseatic cities (see Fig. 1).

In this case study, the sculpture of St. Gertrude of Nivelles as analysis object was selected (see Fig. 2), and the interesting results obtained from the analysis of the chemical composition of the blackish ornament on the golden dress are discussed. More information about the investigation of the other painting materials of the sculpture of Gertrude can be found in the book called „Rode Altarpiece in Close-up” ( available also in Moodle) [1].

As seen in Fig. 2, the sculpture of Gertrude is partially conserved (that was made in the Soviet Union period), and besides the original material also the used conservation materials were determined.
At first, on the site at St. Nicholas’ Church, on the sculpture, non-destructive elemental analysis of the materials with portable ED-XRF was carried out by Dr Riin Rebane from the Estonian Environmental Research Centre. During the portative ED-XRF analysis, on the blackish ornament of Gertrude’s dress, mainly copper (Cu), however also lower amounts of iron and calcium were detected (see Figs. 2 and 3).
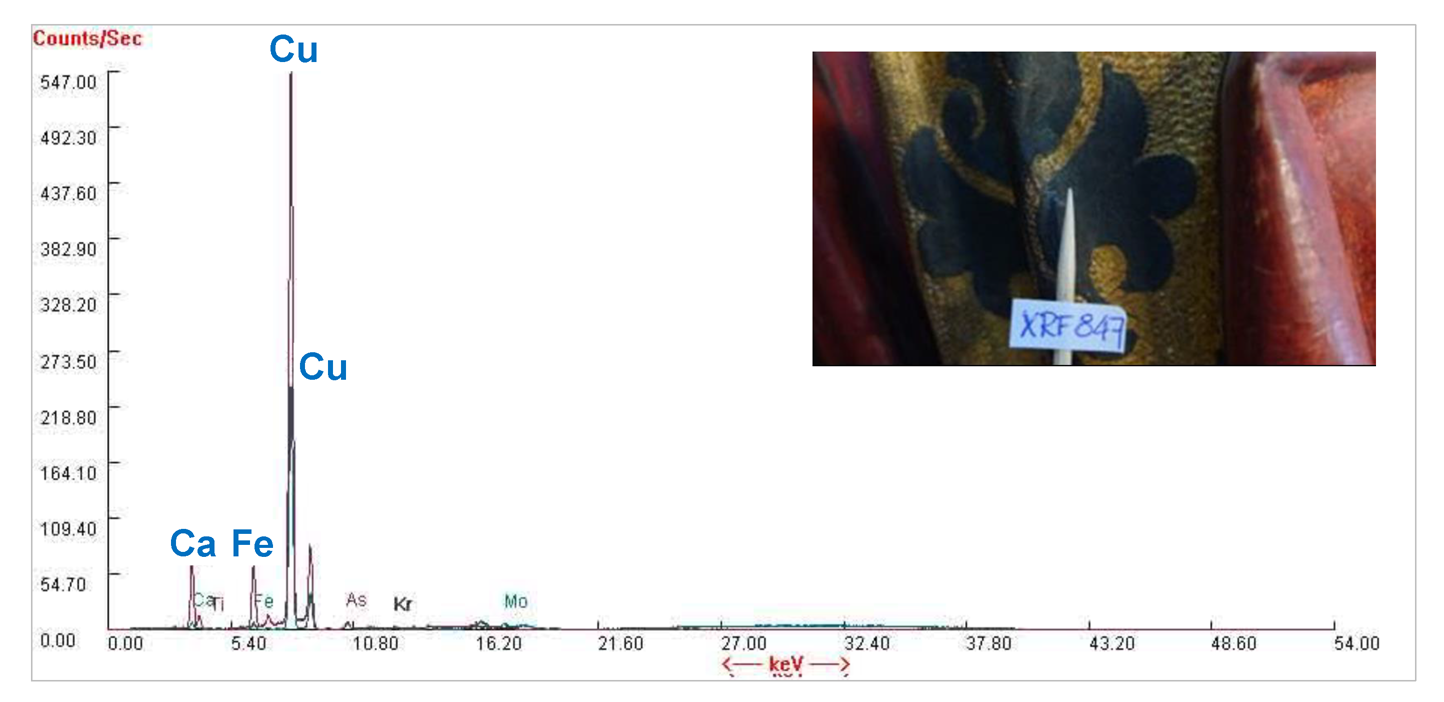
This find was a surprise because copper is not in the composition of any known black (or dark brown) pigment. However, both green and blue pigments do contain copper. This indicates the possibility that this layer may have been darkened over time or that a green or blue layer was under this layer, and the blackish layer could have been added later.
To get more information, further analysis was needed with more sophisticated analytical instruments at the University of Tartu laboratories. Small sample pieces from the sculpture were taken to continue the investigation (see Fig. 2).
2. Used analytical techniques
While the taken sample pieces were tiny, the instrumental techniques were used in specific order to avoid any waste of the sample. Analytical techniques were used in the following order:
- An optical microscope was used to determine the layered structure (stratigraphy) of the paint sample. For that, a cross-section block of the sample piece was made. For preparing a paint cross-section block, the paint sample piece was embedded entirely into a liquid polymer (Technovit 2000 LC) in the mould, then the mould was placed in the visible blue-light polymerization chamber (Technotray CU), and after about 30 min a hardened cross-section block was obtained. The cross-section block was polished with a polishing device (MetaServ 250/ Vector Power Head) using different abrasive papers until it was as smooth as glass. The paint pieces and cross-section block were observed under the Leica M165 FC stereomicroscope.
- ATR-FT-IR spectrometer was selected because this technique does not require any sample preparation, tiny samples can be analysed in the broad wavenumber range (4000-2225 cm-1) and determine the chemical composition of all the paint components (pigment, filler, binder, additives). For the analysis, paint layers were separated manually with a sharp scalpel under the optical microscope. Then the investigation was carried out using Thermo Scientific Nicolet 6700 FT-IR spectrometer with Smart Orbit diamond micro-ATR accessory. In this research, we could not use an ATR-FT-IR microspectrometer (or ATR-FT-IR microscope) to analyse paint layers directly on the paint cross-section because there was an interfering polymer on the paint layers which was not possible to remove. If we had more polished the cross-section block, we would have lost a tiny paint sample piece.
- SEM-EDS technique for the elemental analysis was used to confirm the compositions of the pigment and filler in the paint. For the analysis, an uncoated paint sample was placed onto a sticky carbon conductive tab and measurements were conducted using variable pressure Zeiss EVO MA15 scanning electron microscope (SEM) equipped with Oxford X-MAX energy-dispersive detector (EDS). The analysis was carried out at the Department of Geology, University of Tartu.
3. Results
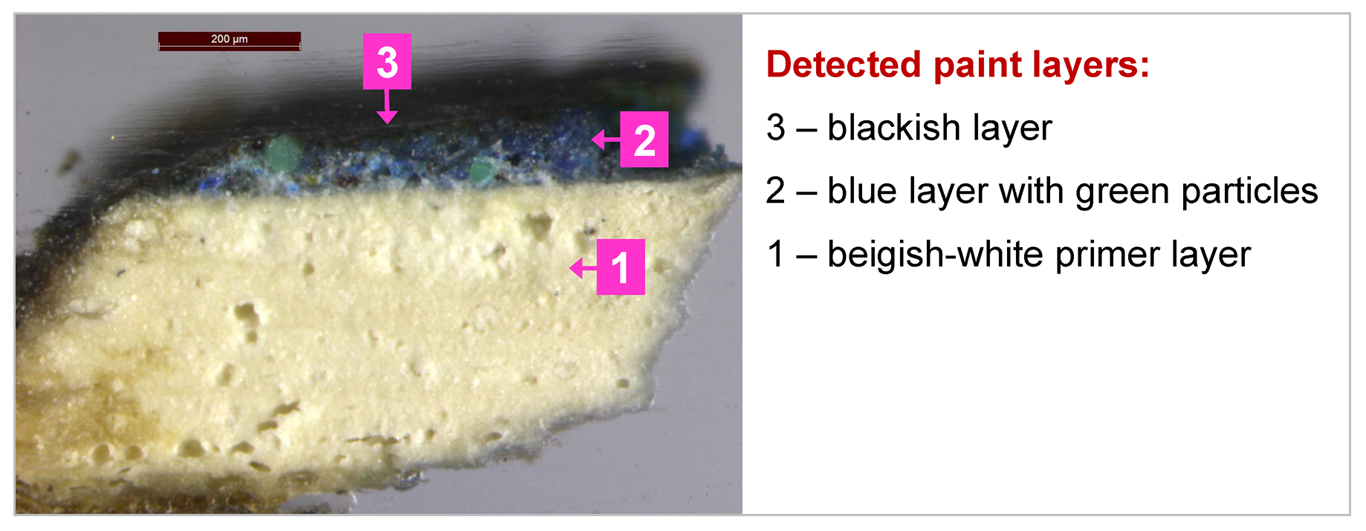
Analysis with an optical microscope
The cross-section block was examined under the Leica stereomicroscope. Fig. 4. shows well that under the blackish layer there is a blue layer. Based on that, we can assume that with ED-XRF detected copper (Cu) could belong to the blue paint.
Analysis with ATR-FT-IR spectrometer
The investigation of the chemical composition of paint layers continued with ATR-FT-IR spectrometer. Fig. 5 presents ATR-FT-IR spectra of all the manually separated paint layers of the sample and Table 1 interpretation of these spectra.
Two sample pieces – one sticky material and the other blackish piece – from the upper layer were analysed with ATR-FT-IR. IR spectra indicate that sticky material contains polyvinyl acetate (PVAc) and calcium carbonate as an additive. With this analysis, one of the conservation materials that was used in the earlier conservation works was determined. ATR-FT-IR spectrum of blackish sample piece contains some silicates, calcium carbonate, bands that belong to protein and some ester-containing material (maybe oil and traces of PVAc). These results indicate that the blackish upper layer could be a tempera-oil-containing paint layer.
As can be seen in the microscopic image (see Fig. 4), under the blackish layer there is a bright blue layer with light green particles. ATR-FT-IR spectrum of the blue layer indicates that it contains azurite as a pigment, silicates and calcium carbonate as fillers (or additives) and a protein-based binder (tempera paint). Under the blue layer is a chalk-containing primer with a small amount of silicates as additives and protein binder.
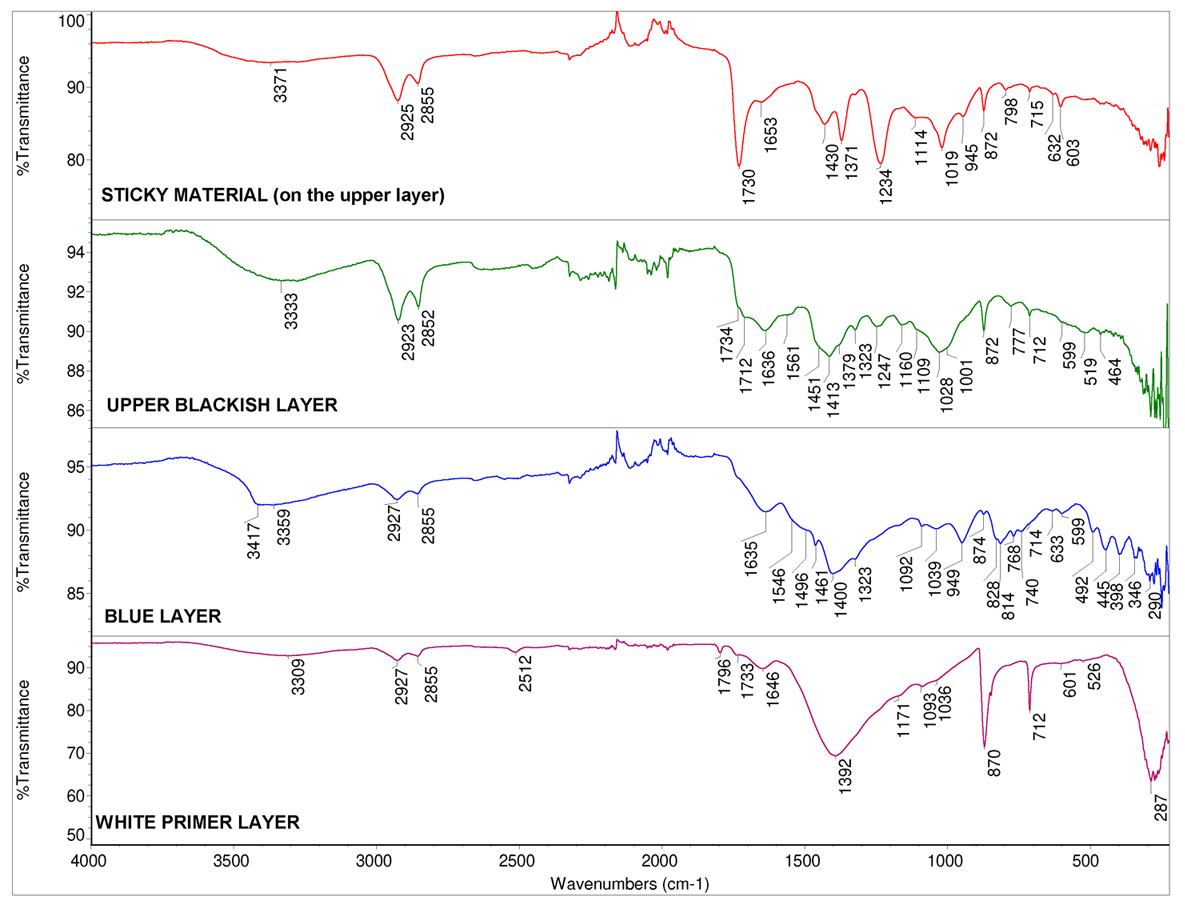
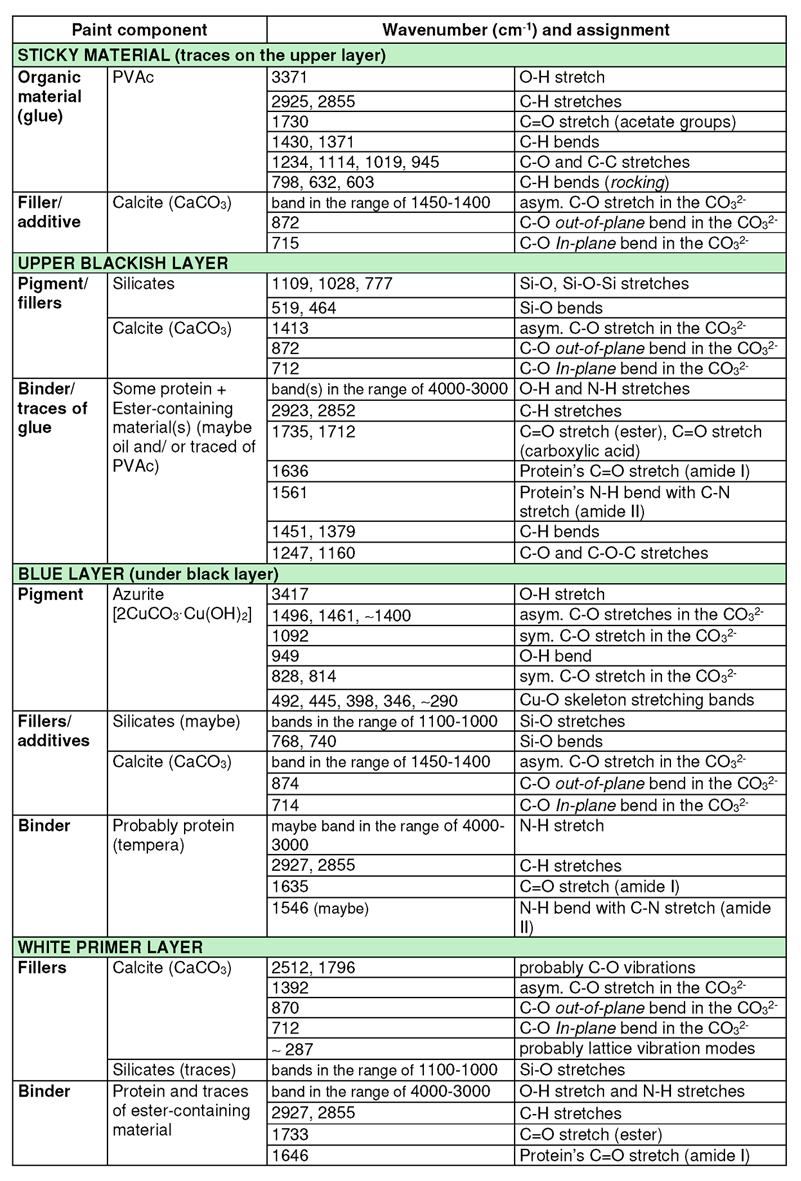
Table 1. Interpretation of the ATR-FT-IR spectra of the paint layers [2–6].
Analysis with SEM-EDS
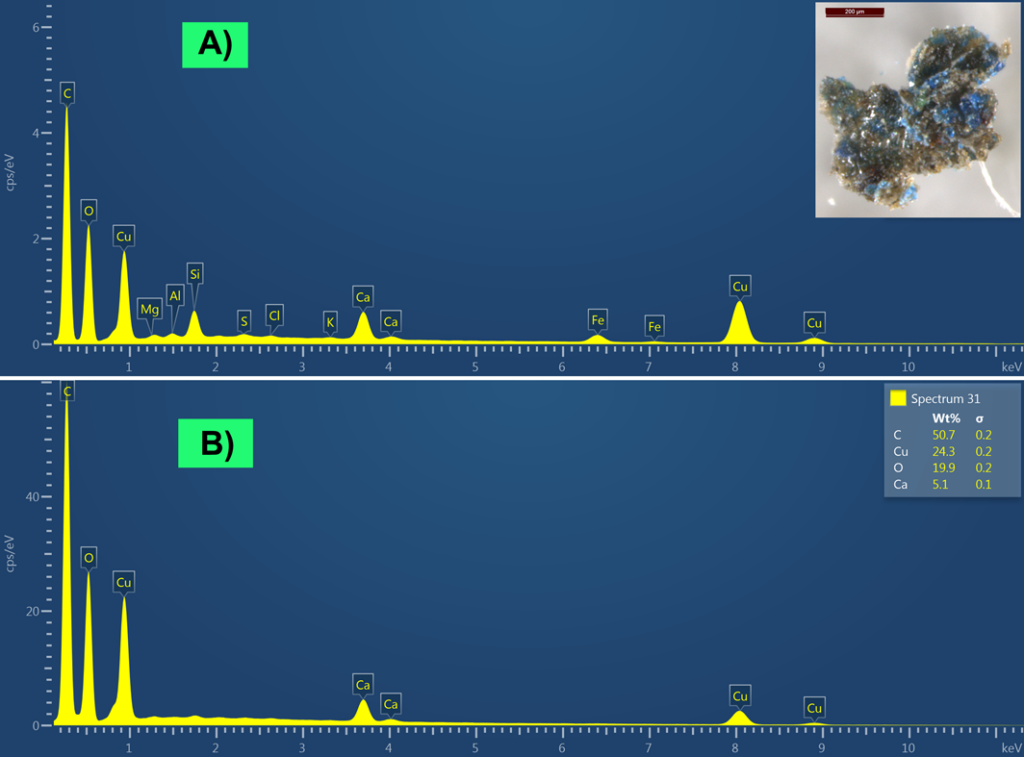
For confirmation of the compositions of the blackish material and blue paint under that, SEM-EDS analysis was carried out. Fig. 6 presents two SEM-EDS spectra – the first spectrum was measured from the larger area on the sample piece (blue particles were together with an upper dark brownish-blackish material), and the second spectrum was measured directly from the blue particles. Both SEM-EDS spectra contain intensive Cu peaks, which belong to the composition of the blue paint and confirm the presence of azurite (2CuCO3·Cu(OH)2) as the blue pigment. Other elements (Fe, Si, Al, Mg) in the first EDS spectrum (see Fig. 6, spectrum A) probably belong to the dark brownish-blackish material and may belong to the composition of brown ochre (Fe2O3+silicates). A more blackish tone may come from carbon (C), however we cannot rule out the possibility that the blackish colour may come from magnetite (Fe3O4). Both SEM-EDS spectra also contain Ca, which may belong to chalk (CaCO3).
4. Conclusions
This investigation shows well how important it is to carry out analysis with multiple methods. Using different analytical techniques, we discovered that originally the ornaments on the golden dress (containing pure gold with traces of silver) of the sculpture of St. Gertrude of Nivelles were made with beautiful azurite blue tempera paint. The results indicate (detection of Fe, Si, Al, Mg with SEM-EDS) that in some earlier times, the ornaments could have been over-painted with dark brownish-blackish paint (which may contain brown ochre with additives). However, we cannot rule out the possibility of darkening the coating layer that was applied to the surface at some period (or darkening of azurite-containing paint layer), and traces of detected elements may come from other layers or impurities. In any case, there is an interesting puzzle that needs further investigation. Additionally, valuable information about conservation materials (PVAc adhesive or its emulsion was determined) was also obtained, which could be applied during conservation works made in the Soviet Union period.
- Rode altar lähivaates =: Rode altarpiece in close-up; Hiiop, H., Kurisoo, M., Eds.; Eesti Kunstimuuseum: Tallinn, 2016.
- Vahur, S.; Teearu, A.; Peets, P.; Joosu, L.; Leito, I. ATR-FT-IR Spectral Collection of Conservation Materials in the Extended Region of 4000-80 cm–1. Anal. Bioanal. Chem. 2016, 408 (13), 3373–3379. https://doi.org/10.1007/s00216-016-9411-5.
- Wei, S.; Pintus, V.; Schreiner, M. Photochemical Degradation Study of Polyvinyl Acetate Paints Used in Artworks by Py–GC/MS. J. Anal. Appl. Pyrolysis 2012, 97, 158–163. https://doi.org/10.1016/j.jaap.2012.05.004.
- França De Sá, S.; Viana, C.; Ferreira, J. L. Tracing Poly(Vinyl Acetate) Emulsions by Infrared and Raman Spectroscopies: Identification of Spectral Markers. Polymers 2021, 13 (21), 3609. https://doi.org/10.3390/polym13213609.
- Vahur, S.; Kiudorv, L.; Somelar, P.; Cayme, J.-M.; Retrato, M. D. C.; Remigio, R. J.; Sharma, V.; Oras, E.; Leito, I. Quantitative Mineralogical Analysis of Clay-Containing Materials Using ATR-FT-IR Spectroscopy with PLS Method. Anal. Bioanal. Chem. 2021, 413 (26), 6535–6550. https://doi.org/10.1007/s00216-021-03617-9.
- Derrick, M. R.; Stulik, D.; Landry, J. M. Infrared Spectroscopy in Conservation Science; Scientific tools for conservation; Getty Conservation Institute: Los Angeles, 1999.


