MOOC: Validation of liquid chromatography mass spectrometry (LC-MS) methods (analytical chemistry) course
8. Stability
In this part of the course the issues related to the analyte’s (insufficient) are introduced and explained. In addition, different approaches are proposed how to deal with unstable analytes.
Stability introduction
http://www.uttv.ee/naita?id=23632
https://www.youtube.com/watch?v=1XaPGinpkfQ&feature=youtu.be
Analyte stability is universally not included in the validation guidelines as a validation parameter (it is included only in SANTE/SANCO, EU regulation 2021/808, EMA, and ). The reason for this is that if the analyte is unstable, its decomposition influences the and of the procedure and is, thus, accounted for by these two parameters. However, stability is a very important parameter in bioanalytics and this importance justifies considering it as a separate performance parameter. In bioanalytics the instability of an analyte is rather a rule, not an exception. Therefore, it is necessary to find out whether the analyte is unstable, how unstable it is, could we change the conditions in our method to improve analytes’ stability and, finally, how can we still get acceptable results if an analyte is unstable.
Analyte stability must be ensured during the sample collection, processing, storage, handling, extraction and duration of the analysis in order to generate reliable (bio)analytical data. Therefore, the stability tests can be among the most time-consuming tests in the validation procedure.
Stability is the lowering of the analyte content in the sample over period of time.
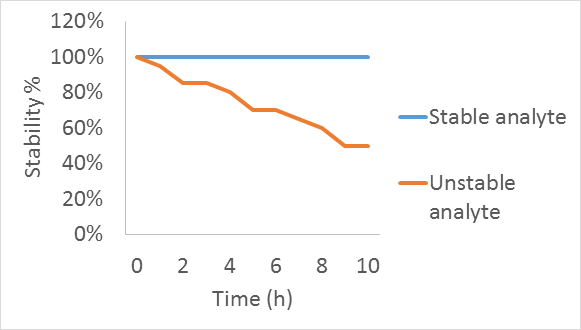
Figure 1. Analyte stability over the period of time.
If an analyte is stable, then the concentration remains the same in time e. g. 100%. If an analyte degrades with time, then its concentration is decreased and also the stability is lower than 100%.
Decomposition usually leads to a decrease in analyte content. However, in the case of analysis of decomposition products, degradation can lead to an increase in the analyte content.
If an analyte is unstable, its decomposition influences the trueness and precision (since both systematic and random effects are usually involved) of the procedure and is, thus, indirectly accounted for by these two parameters. It is nevertheless useful to handle analyte stability separately from trueness and precision.
The rate of decomposition can be strongly dependent on the minor experimental details (type of matrix, access of oxygen, temperature, light etc.).
Furthermore, besides the analyte in the samples, analyte in the standards can also decompose. If both occur at the same rate, the decomposition only affects precision. If not, then both trueness and precision are affected. In addition, the EMA guide [] stresses that analyte stability cannot be proven by literature data, further outlining the importance of analyte stability testing.

